J Vet Sci.
2017 Sep;18(3):283-289. 10.4142/jvs.2017.18.3.283.
Survey of porcine respiratory disease complex-associated pathogens among commercial pig farms in Korea via oral fluid method
- Affiliations
-
- 1Department of Infectious Diseases, College of Veterinary Medicine, Konkuk University, Seoul 05029, Korea.
- 2Veterinary Epidemiology Division, Animal and Plant Quarantine Agency, Gimcheon 39660, Korea. vet10@naver.com
- KMID: 2412445
- DOI: http://doi.org/10.4142/jvs.2017.18.3.283
Abstract
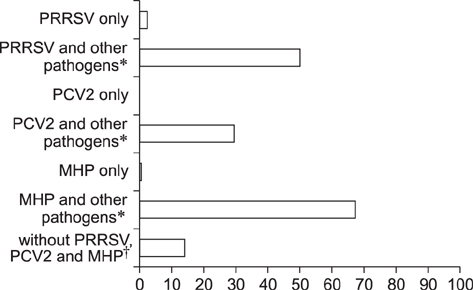
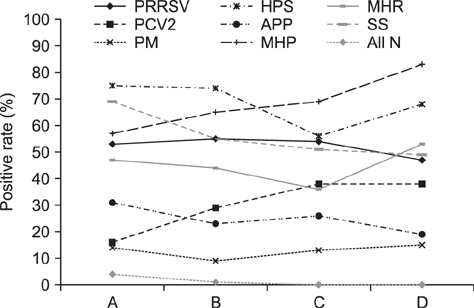
- Oral fluid analysis for herd monitoring is of interest to the commercial pig production in Korea. The aim of this study was to investigate pathogen-positive rates and correlations among eight pathogens associated with porcine respiratory disease complex by analyzing oral fluid samples from 214 pig groups from 56 commercial farms. Samples collected by a rope-chewing method underwent reverse-transcriptase polymerase chain reaction (RT-PCR) or standard polymerase chain reaction (PCR) analysis, depending on the microorganism. Pathogens were divided into virus and bacteria groups. The former consisted of porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 (PCV2), and the latter Pasteurella multocida, Haemophilus parasuis, Actinobacillus pleuropneumoniae, Mycoplasma hyopneumoniae (MHP), Mycoplasma hyorhinis, and Streptococcus suis (SS). All pathogens were detected more than once by PCR. Age-based analysis showed the PCR-positive rate increased with increasing age for PCV2 and MHP, whereas SS showed the opposite. Correlations between pathogens were assessed among 36 different pair combinations; only seven pairs showed statistically significant correlations. In conclusion, the oral fluid method could be a feasible way to detect various swine respiratory disease pathogens and, therefore, could complement current monitoring systems for respiratory diseases in the swine industry.
Keyword
MeSH Terms
-
Animals
Farms/statistics & numerical data
Mouth/virology
Porcine Reproductive and Respiratory Syndrome/*epidemiology/virology
*Porcine respiratory and reproductive syndrome virus
Republic of Korea/epidemiology
Reverse Transcriptase Polymerase Chain Reaction/veterinary
Surveys and Questionnaires
Swine/virology
Figure
Cited by 1 articles
-
Age-specific Prevalence of Porcine Reproductive and Respiratory Syndrome Virus, Porcine Circovirus Type 2, and
Mycoplasma hyopneumoniae in Korea Pig Farms
Ikjae Kang, Heejin Ham
J Bacteriol Virol. 2020;50(1):9-16. doi: 10.4167/jbv.2020.50.1.009.
Reference
-
1. Chae C. Porcine circovirus type 2 and its associated diseases in Korea. Virus Res. 2012; 164:107–113.
Article2. Choi EJ, Lee CH, Song JY, Song HJ, Park CK, Kim B, Shin YK. Genetic diversity of porcine reproductive and respiratory syndrome virus in Korea. J Vet Sci. 2013; 14:115–124.
Article3. Dorr PM, Baker RB, Almond GW, Wayne SR, Gebreyes WA. Epidemiologic assessment of porcine circovirus type 2 coinfection with other pathogens in swine. J Am Vet Med Assoc. 2007; 230:244–250.
Article4. Fablet C, Marois C, Dorenlor V, Eono F, Eveno E, Jolly JP, Le Devendec L, Kobisch M, Madec F, Rose N. Bacterial pathogens associated with lung lesions in slaughter pigs from 125 herds. Res Vet Sci. 2012; 93:627–630.
Article5. Fablet C, Marois C, Kuntz-Simon G, Rose N, Dorenlor V, Eono F, Eveno E, Jolly JP, Le Devendec L, Tocqueville V, Quéguiner S, Gorin S, Kobisch M, Madec F. Longitudinal study of respiratory infection patterns of breeding sows in five farrow-to-finish herds. Vet Microbiol. 2011; 147:329–339.
Article6. Fablet C, Marois-Créhan C, Simon G, Grasland B, Jestin A, Kobisch M, Madec F, Rose N. Infectious agents associated with respiratory diseases in 125 farrow-to-finish pig herds: a cross-sectional study. Vet Microbiol. 2012; 157:152–163.
Article7. Fraile L, Alegre A, López-Jiménez R, Nofrarías M, Segalés J. Risk factors associated with pleuritis and cranio-ventral pulmonary consolidation in slaughter-aged pigs. Vet J. 2010; 184:326–333.
Article8. Gram T, Ahrens P. Improved diagnostic PCR assay for Actinobacillus pleuropneumoniae based on the nucleotide sequence of an outer membrane lipoprotein. J Clin Microbiol. 1998; 36:443–448.
Article9. Hälli O, Ala-Kurikka E, Nokireki T, Skrzypczak T, Raunio-Saarnisto M, Peltoniemi OAT, Heinonen M. Prevalence of and risk factors associated with viral and bacterial pathogens in farmed European wild boar. Vet J. 2012; 194:98–101.
Article10. Hansen MS, Pors SE, Jensen HE, Bille-Hansen V, Bisgaard M, Flachs EM, Nielsen OL. An investigation of the pathology and pathogens associated with porcine respiratory disease complex in Denmark. J Comp Pathol. 2010; 143:120–131.
Article11. Hričínová M, Holoda E, Mudroňová D, Ondrašovičová S. Multiplex PCR assay for detection of Actinobacillus pleuropneumoniae, Pasteurella multocida and Haemophilus parasuis in lungs of pigs from a slaughterhouse. Folia Microbiol (Praha). 2010; 55:635–640.
Article12. Jones TH, Muehlhauser V. Effect of handling and storage conditions and stabilizing agent on the recovery of viral RNA from oral fluid of pigs. J Virol Methods. 2014; 198:26–31.
Article13. Kerdsin A, Dejsirilert S, Akeda Y, Sekizaki T, Hamada S, Gottschalk M, Oishi K. Fifteen Streptococcus suis serotypes identified by multiplex PCR. J Med Microbiol. 2012; 61:1669–1672.14. Kittawornrat A, Prickett J, Chittick W, Wang C, Engle M, Johnson J, Patnayak D, Schwartz T, Whitney D, Olsen C, Schwartz K, Zimmerman J. Porcine reproductive and respiratory syndrome virus (PRRSV) in serum and oral fluid samples from individual boars: will oral fluid replace serum for PRRSV surveillance? Virus Res. 2010; 154:170–176.
Article15. Kittawornrat A, Prickett J, Wang C, Olsen C, Irwin C, Panyasing Y, Ballagi A, Rice A, Main R, Johnson J, Rademacher C, Hoogland M, Rowland R, Zimmerman J. Detection of Porcine reproductive and respiratory syndrome virus (PRRSV) antibodies in oral fluid specimens using a commercial PRRSV serum antibody enzyme-linked immunosorbent assay. J Vet Diagn Invest. 2012; 24:262–269.
Article16. MacInnes JI, Gottschalk M, Lone AG, Metcalf DS, Ojha S, Rosendal T, Watson SB, Friendship RM. Prevalence of Actinobacillus pleuropneumoniae, Actinobacillus suis, Haemophilus parasuis, Pasteurella multocida, and Streptococcus suis in representative Ontario swine herds. Can J Vet Res. 2008; 72:242–248.17. Marois C, Cariolet R, Morvan H, Kobisch M. Transmission of pathogenic respiratory bacteria to specific pathogen free pigs at slaughter. Vet Microbiol. 2008; 129:325–332.
Article18. Moorkamp L, Nathues H, Spergser J, Tegeler R, Grosse Beilage E. Detection of respiratory pathogens in porcine lung tissue and lavage fluid. Vet J. 2008; 175:273–275.
Article19. O'Sullivan T, Friendship R, Blackwell T, Pearl D, McEwen B, Carman S, Slavić D, Dewey C. Microbiological identification and analysis of swine tonsils collected from carcasses at slaughter. Can J Vet Res. 2011; 75:106–111.20. Oliveira S, Galina L, Pijoan C. Development of a PCR test to diagnose Haemophilus parasuis infections. J Vet Diagn Invest. 2001; 13:495–501.
Article21. Olsen C, Karriker L, Wang C, Binjawadagi B, Renukaradhya G, Kittawornrat A, Lizano S, Coetzee J, Main R, Meiszberg A, Panyasing Y, Zimmerman J. Effect of collection material and sample processing on pig oral fluid testing results. Vet J. 2013; 198:158–163.
Article22. Olsen C, Wang C, Christopher-Hennings J, Doolittle K, Harmon KM, Abate S, Kittawornrat A, Lizano S, Main R, Nelson E a, Otterson T, Panyasing Y, Rademacher C, Rauh R, Shah R, Zimmerman J. Probability of detecting Porcine reproductive and respiratory syndrome virus infection using pen-based swine oral fluid specimens as a function of within-pen prevalence. J Vet Diagn Invest. 2013; 25:328–335.
Article23. Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and Porcine circovirus type 2. Vet Pathol. 2004; 41:624–640.
Article24. Palzer A, Ritzmann M, Wolf G, Heinritzi K. Associations between pathogens in healthy pigs and pigs with pneumonia. Vet Rec. 2008; 162:267–271.
Article25. Pogranichniy RM, Yoon KJ, Harms PA, Sorden SD, Daniels M. Case-control study on the association of Porcine circovirus type 2 and other swine viral pathogens with postweaning multisystemic wasting syndrome. J Vet Diagn Invest. 2002; 14:449–456.
Article26. Prickett J, Kim W, Simer R, Yoon KJ, Zimmerman J. Oral-fluid samples for surveillance of commercial growing pigs for porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 infections. J Swine Health Prod. 2008; 16:86–91.27. Prickett J, Simer R, Christopher-Hennings J, Yoon KJ, Evans RB, Zimmerman JJ. Detection of Porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: a longitudinal study under experimental conditions. J Vet Diagn Invest. 2008; 20:156–163.
Article28. Ramirez A, Wang C, Prickett JR, Pogranichniy R, Yoon KJ, Main R, Johnson JK, Rademacher C, Hoogland M, Hoffmann P, Kurtz A, Kurtz E, Zimmerman J. Efficient surveillance of pig populations using oral fluids. Prev Vet Med. 2012; 104:292–300.
Article29. Shin J, Bautista EM, Kang YB, Molitor TW. Quantitation of porcine reproductive and respiratory syndrome virus RNA in semen by single-tube reverse transcription-nested polymerase chain reaction. J Virol Methods. 1998; 72:67–79.
Article30. Stakenborg T, Vicca J, Butaye P, Imberechts H, Peeters J, de Kruif A, Haesebrouck F, Maes D. A multiplex PCR to identify porcine mycoplasmas present in broth cultures. Vet Res Commun. 2006; 30:239–247.
Article31. Wieland B, Werling D, Nevel A, Rycroft A, Demmers TG, Wathes CM, Grierson S, Cook AJC, Done SH, Armstrong D. Porcine circovirus type 2 infection before and during an outbreak of postweaning multisystemic wasting syndrome on a pig farm in the UK. Vet Rec. 2012; 170:596.
Article32. Wongnarkpet S, Pfeiffer DU, Morris RS, Fenwick SG. An on-farm study of the epidemiology of Actinobacillus pleuropneumoniae infection in pigs as part of a vaccine efficacy trial. Prev Vet Med. 1999; 39:1–11.
Article33. Zhou JY, Chen QX, Ye JX, Shen HG, Chen TF, Shang SB. Serological investigation and genomic characterization of PCV2 isolates from different geographic regions of Zhejiang province in China. Vet Res Commun. 2006; 30:205–220.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Survey of porcine respiratory disease complex-associated pathogens among commercial pig farms in Korea via oral fluid method
- Age-specific Prevalence of Porcine Reproductive and Respiratory Syndrome Virus, Porcine Circovirus Type 2, and Mycoplasma hyopneumoniae in Korea Pig Farms
- First detection and genetic characterization of porcine parvovirus 7 from Korean domestic pig farms
- Genetic diversity of porcine reproductive and respiratory syndrome virus in Korea
- Acute porcine reproductive and respiratory syndrome outbreaksin immunized sow herds: from occurrence to stabilization under whole herd vaccination strategy