J Vet Sci.
2012 Jun;13(2):111-118. 10.4142/jvs.2012.13.2.111.
Isolation and genetic characterization of Japanese encephalitis virus from equines in India
- Affiliations
-
- 1National Research Centre on Equines, Sirsa Road, Hisar-125001, Haryana, India. brgulati@gmail.com
- KMID: 1376184
- DOI: http://doi.org/10.4142/jvs.2012.13.2.111
Abstract
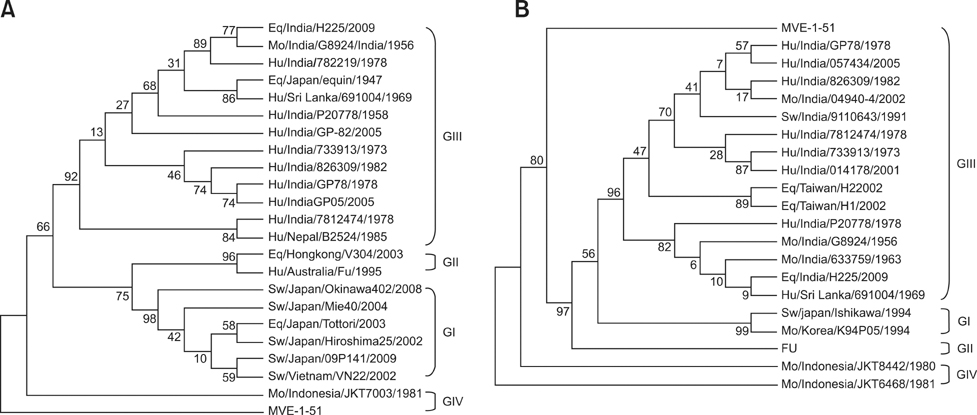
- Japanese encephalitis (JE) is an important vector-borne viral disease of humans and horses in Asia. JE outbreaks occur regularly amongst humans in certain parts of India and sporadic cases occur among horses. In this study, JE seroprevalence and evidence of JE virus (JEV) infection among horses in Haryana (India) is described. Antibodies against JEV were detected in 67 out of 637 (10.5%) horses screened between 2006 and 2010. Two foals exhibiting neurological signs were positive for JEV RNA by RT-PCR; JEV was isolated from the serum of one of the foals collected on the second day of illness. This is the first report of JEV isolation from a horse in India. Furthermore, a pool of mosquitoes collected from the premises housing these foals was positive for JEV RNA by RT-PCR. Three structural genes, capsid (C), premembrane (prM), and envelope (E) of the isolated virus (JE/eq/India/H225/2009) spanning 2,500 nucleotides (from 134 to 2,633) were cloned and sequenced. BLAST results showed that these genes had a greater than 97% nucleotide sequence identity with different human JEV isolates from India. Phylogenetic analysis based on E- and C/prM genes indicated that the equine JEV isolate belonged to genotype III and was closely related to the Vellore group of JEV isolates from India.
MeSH Terms
-
Animals
Antibodies, Monoclonal
Cloning, Molecular
Culex/virology
Encephalitis Virus, Japanese/*genetics/*isolation & purification
Encephalitis, Japanese/epidemiology/*veterinary/virology
Enzyme-Linked Immunosorbent Assay/methods/veterinary
Female
Genes, Viral
Genotype
Horse Diseases/epidemiology/*virology
Horses
India/epidemiology
RNA, Viral/genetics/isolation & purification
Reverse Transcriptase Polymerase Chain Reaction/veterinary
Seroepidemiologic Studies
Figure
Cited by 2 articles
-
Detection of Neutralizing Antibody Against Japanese Encephalitis Virus in Wild Boars of Korea
Dong-Kun Yang, Ha-Hyun Kim, Bang-Hun Hyun, Seong-In Lim, Yun-Kyoung Nam, Jin-Ju Nah, Jae-Young Song
J Bacteriol Virol. 2012;42(4):353-356. doi: 10.4167/jbv.2012.42.4.353.Establishment of a Multiplex RT-PCR for the Sensitive and Differential Detection of Japanese Encephalitis Virus Genotype 1 and 3
Dong-Kun Yang, Ha-Hyun Kim, Hyun-Ye Jo, Sung-Suk Choi, In-Soo Cho
J Bacteriol Virol. 2016;46(4):231-238. doi: 10.4167/jbv.2016.46.4.231.
Reference
-
1. Alka , Bharati K, Malik YPS, Vrati S. Immunogenicity and protective efficacy of the E. coli-expressed domain III of Japanese encephalitis virus envelope protein in mice. Med Microbiol Immunol. 2007. 196:227–231.
Article2. Chakravarti SK, Sardana DN, Mukherjee KK, Mitra AC, Chakrabarty MS. Serological evidence of infection with Japanese encephalitis virus in mules of eastern Himalayan region. Indian J Med Res. 1981. 73:4–7.3. Chen WR, Rico-Hesse R, Tesh RB. A new genotype of Japanese encephalitis virus from Indonesia. Am J Trop Med Hyg. 1992. 47:61–69.
Article4. Chen WR, Tesh RB, Rico-Hesse R. Genetic variation of Japanese encephalitis virus in nature. J Gen Virol. 1990. 71(Pt 12):2915–2922.
Article5. Chung YJ, Nam JH, Ban SJ, Cho HW. Antigenic and genetic analysis of Japanese encephalitis viruses isolated from Korea. Am J Trop Med Hyg. 1996. 55:91–97.
Article6. Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958. 7:561–573.
Article7. Das BP, Lal S, Saxena VK. Outdoor resting preference of Culex tritaeniorhynchus, the vector of Japanese encephalitis in Warangal and Karim Nagar districts, Andhra Pradesh. J Vector Borne Dis. 2004. 41:32–36.8. Dhillon GP, Raina VK. Epidemiology of Japanese encephalitis in context with Indian scenario. J Indian Med Assoc. 2008. 106:660–663.9. D'Souza MB, Nagarkatti S, Rao KM. Serological evidence for arboviral infection among horses--HI test by filter paper disc method. Indian J Med Res. 1978. 67:708–712.10. Fulmali PV, Sapkal GN, Athawale S, Gore MM, Mishra AC, Bondre VP. Introduction of Japanese Encephalitis virus genotype I, India. Emerg Infect Dis. 2011. 17:319–321.
Article11. Ghosh D, Basu A. Japanese encephalitis-a pathological and clinical perspective. PLoS Negl Trop Dis. 2009. 3:e437.
Article12. Gould DJ, Byrne RJ, Hayes DE. Experimental infection of horses with Japanese encephalitis virus by mosquito bites. Am J Trop Med Hyg. 1964. 13:742–746.
Article13. Gowal D, Singh G, Gowal KN, Bhau LN, Saxena SN. Serological evidence of Japanese encephalitis virus infection in equines of North Himalayan region. Indian Vet J. 1990. 67:398–401.14. Hasegawa H, Yoshida M, Fujita S, Kobayashi Y. Comparison of structural proteins among antigenically different Japanese encephalitis virus strains. Vaccine. 1994. 12:841–844.
Article15. Ihara T, Kano R, Nakajima Y, Sugiura T, Imagawa H, Izuchi T, Samejima T. Detection of antibody to Japanese encephalitis virus (JEV) by enzyme-linked immunosorbent assay (ELISA). J Equine Sci. 1997. 8:25–28.
Article16. Johnson AJ, Martin DA, Karabatsos N, Roehrig JT. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J Clin Microbiol. 2000. 38:1827–1831.
Article17. Kar NJ, Saxena VK. Some epidemiological characteristics of Japanese encephalitis in Haryana state of northern India. J Commun Dis. 1998. 30:129–131.18. Katyal R, Bhardwaj M, Harit AK, Sharma SK, Kumar K, Gill KS. Dengue, Japanese encephalitis and West Nile flaviviral infections detected during a dengue outbreak in sonepat district, Haryana state, India. Dengue Bull. 2000. 24:24–28.19. Konishi E, Shoda M, Ajiro N, Kondo T. Development and evaluation of an enzyme-linked immunosorbent assay for quantifying antibodies to Japanese encephalitis virus nonstructural 1 protein to detect subclinical infections in vaccinated horses. J Clin Microbiol. 2004. 42:5087–5093.
Article20. Lam KHK, Ellis TM, Williams DT, Daniels PW, Riggs CM. Japanese encephalitis in racing thoroughbred gelding in Hong Kong. Vet Rec. 2005. 157:168–173.21. Lian WC, Liau MY, Mao CL. Diagnosis and genetic analysis of Japanese encephalitis virus infected in horses. J Vet Med B Infect Dis Vet Public Health. 2002. 49:361–365.
Article22. Ma SP, Yoshida Y, Makino Y, Tadano M, Ono T, Ogawa M. Short report: a major genotype of Japanese encephalitis virus currently circulating in Japan. Am J Trop Med Hyg. 2003. 69:151–154.
Article23. Mall MP, Kumar A, Malik SV. Sero-positivity of domestic animals against Japanese encephalitis in Bareilly area, U.P. J Commun Dis. 1995. 27:242–246.24. Misra UK, Kalita J. Overview: Japanese encephalitis. Prog Neurobiol. 2010. 91:108–120.
Article25. Nga PT, del Carmen Parquet M, Cuong VD, Ma SP, Hasebe F, Inoue S, Makino Y, Takagi M, Nam VS, Morita K. Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: implications for frequent introductions of JEV from Southeast Asia to East Asia. J Gen Virol. 2004. 85(Pt 6):1625–1631.
Article26. Pant GR. A serological survey of pigs, horses, and ducks in Nepal for evidence of infection with Japanese encephalitis virus. Ann N Y Acad Sci. 2006. 1081:124–129.
Article27. Parida M, Dash PK, Tripathi NK, Ambuj , Sannarangaiah S, Saxena P, Agarwal S, Sahni AK, Singh SP, Rathi AK, Bhargava R, Abhyankar A, Verma SK, Rao PVL, Sekhar K. Japanese encephalitis outbreak, India, 2005. Emerg Infect Dis. 2006. 12:1427–1430.
Article28. Rai GP, Tuteja U, Kumar P. Comparison of haemagglutination inhibition and indirect fluorescent antibody tests to detect certain flavivirus antibodies in equines. Acta Microbiol Hung. 1992. 39:69–73.29. Rao P, Joshi K, Mishra B, Ratho RK, Kumar R. An outbreak of Japanese encephalitis in Haryana. J Commun Dis. 2005. 37:78–81.30. Ratho RK, Sethi S, Prasad SR. Prevalence of Japanese encephalitis and West Nile viral infections in pig population in and around Chandigarh. J Commun Dis. 1999. 31:113–116.31. Raut CG, Thakare JP, Padbidri VS, Sapkal GN, Mishra AC, Paramasivan R, Gokhale MD, Mourya DT, Shouche YS, Jayakumar PC. A focal outbreak of Japanese encephalitis among horses in Pune district, India. J Commun Dis. 2003. 35:40–42.32. Schuh AJ, Li L, Tesh RB, Innis BL, Barrett ADT. Genetic characterization of early isolates of Japanese encephalitis virus: genotype II has been circulating since at least 1951. J Gen Virol. 2010. 91(Pt 1):95–102.
Article33. Shankar H, Mall MP, Yadav MP. Seroprevalence of group B arboviruses and equine herpes virus-1 infections in nervous disorders in equines. Indian J Anim Sci. 1988. 58:1–5.34. Sharma SN, Panwar BS. An epidemic of Japanese encephalitis in Haryana in the year 1990. J Commun Dis. 1991. 23:204–205.35. Solomon T, Ni H, Beasley DWC, Ekkelenkamp M, Cardosa MJ, Barrett ADT. Origin and evolution of Japanese encephalitis virus in Southeast Asia. J Virol. 2003. 77:3091–3098.
Article36. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007. 24:1596–1599.
Article37. Uchil PD, Satchidanandam V. Phylogenetic analysis of Japanese encephalitis virus: envelope gene based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent. Am J Trop Med Hyg. 2001. 65:242–251.
Article38. Wang HY, Takasaki T, Fu SH, Sun XH, Zhang HL, Wang ZX, Hao ZY, Zhang JK, Tang Q, Kotaki A, Tajima S, Liang XF, Yang WZ, Kurane I, Liang GD. Molecular epidemiological analysis of Japanese encephalitis virus in China. J Gen Virol. 2007. 88(Pt 3):885–894.
Article39. Widjaja S, Soekotjo W, Hartati S, Jenning GB, Corwin AL. Prevalence of hemagglutination-inhibition and neutralizing antibodies to arboviruses in horses of Java. Southeast Asian J Trop Med Public Health. 1995. 26:109–113.40. Williams DT, Wang LF, Daniels PW, Mackenzie JS. Molecular characterization of the first Australian isolate of Japanese encephalitis virus, the FU strain. J Gen Virol. 2000. 81(Pt 10):2471–2480.
Article41. Yamanaka T, Tsujimura K, Kondo T, Yasuda W, Okada A, Noda K, Okumura T, Matsumura T. Isolation and genetic analysis of Japanese encephalitis virus from a diseased horse in Japan. J Vet Med Sci. 2006. 68:293–295.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serosurveillance for Japanese encephalitis virus infection among equines in India
- Isolation and genetic characterization of Japanese encephalitis virus from equines in India
- Haemagglutination inhibition antibodies of Japanese encephalitis virus to bats, Korea
- The Study of Distribution of HI Antibody Titers Aganist Japanese Encephalitis Virus Among Children in Seoul
- Biophysical characterization of Japanese encephalitis virus (KV1899) isolated from pigs in Korea