J Bacteriol Virol.
2017 Sep;47(3):148-155. 10.4167/jbv.2017.47.3.148.
Indirect ELISA for the Detection of Rabies Virus Antibodies in Dog Sera
- Affiliations
-
- 1Viral Disease Research Division, Animal and Plant Quarantine Agency, MAFRA, Gimcheon, Korea. yangdk@korea.kr
- KMID: 2392963
- DOI: http://doi.org/10.4167/jbv.2017.47.3.148
Abstract
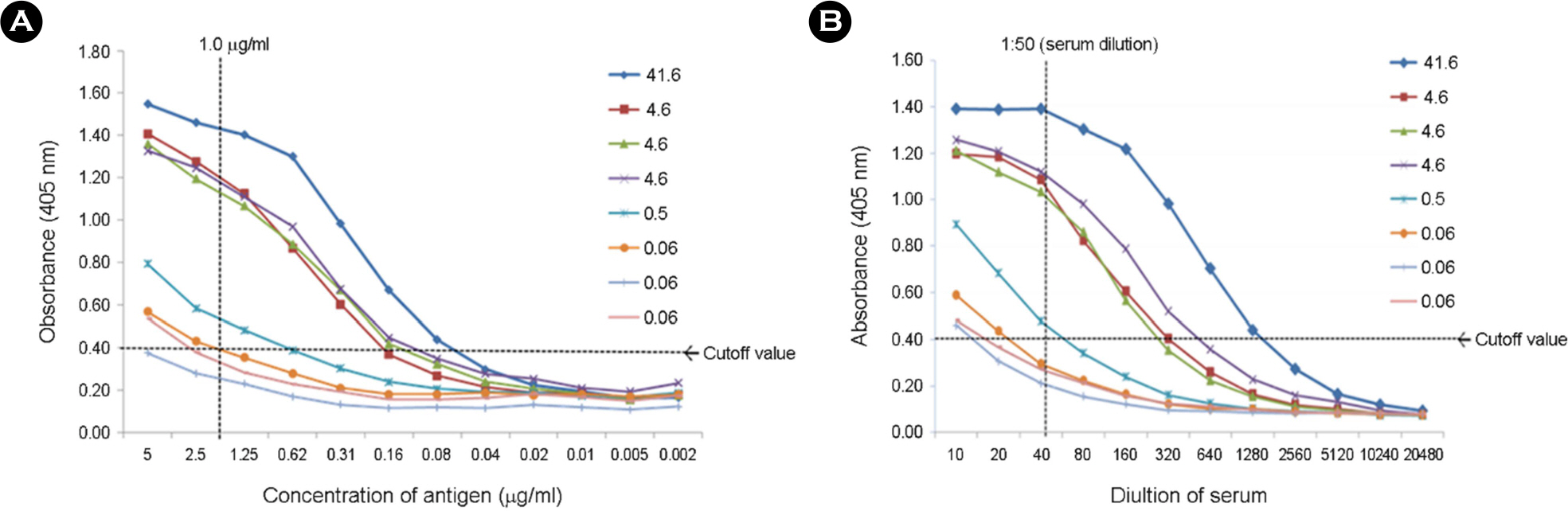
- Rabies is known as the most fatal disease in all warm-blooded animals, including dogs. Among animals that transmit rabies, dogs are mainly responsible for transmitting animal rabies in Asian countries. Detection of rabies virus (RABV) antibodies in dogs is performed by fluorescent antibody virus neutralization (FAVN) test or rapid fluorescent focus inhibition test. These standard assays are difficult to carry out in diagnostic laboratories without sufficient instruments, designated RABV, and cell culture systems. An alternative assay that is easy to conduct and time efficient is required for rapid sero-surveillance following vaccination. Recombinant baculovirus expressing RABV nucleoprotein (RVN) was constructed and the recombinant protein was purified using Ni-NTA and fast protein liquid column chromatography. We developed and evaluated an indirect enzyme-linked immunosorbent assay (I-ELISA) with recombinant RVN for the detection of RABV antibodies in 122 dog serum samples. The I-ELISA results obtained from these samples were compared with FAVN results. The sensitivity, specificity, and accuracy of I-ELISA were 88.1%, 92.5%, and 91.0%, respectively, compared with FAVN. Results of I-ELISA were significantly correlated with that of FAVN (r = 0.81). These results suggest that I-ELISA with recombinant RVN is useful for sero-surveillance of RABV in dog sera.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Development of an Indirect ELISA Featuring Plates Coated with Column Chromatographically Purified Canine Adenovirus Type-1 Antigen
Dong-Kun Yang, Ha-Hyun Kim, Siu Lee, Miryeon Ji, Bok Hee Han, Soobin Oh, Bang-Hun Hyun
J Bacteriol Virol. 2020;50(1):17-24. doi: 10.4167/jbv.2020.50.1.017.
Reference
-
1). Masatani T, Ito N, Shimizu K, Ito Y, Nakagawa K, Sawaki Y, et al. Rabies virus nucleoprotein functions to evade activation of the RIG-I-mediated antiviral response. J Virol. 2010; 84:4002–12.
Article2). Kuzmin IV, Hughes GJ, Rupprecht CE. Phylogenetic relationships of seven previously unclassified viruses within the family Rhabdoviridae using partial nucleoprotein gene sequences. J Gen Virol. 2006; 87:2323–31.
Article3). Katayama S, Yamanaka M, Ota S, Shimizu Y. A New quantitative method for rabies virus by detection of nucleoprotein in virion using ELISA. J Vet Med Sci. 1999; 61:411–6.
Article4). Yang DK, Kim HH, Lee KK, Yoo JY, Seomun H, Cho IS. Mass vaccination has led to the elimination of rabies since 2014 in South Korea. Clin Exp Vaccine Res. 2017; 6:111–9.
Article5). Cheong Y, Kim B, Lee KJ, Park D, Kim S, Kim H, et al. Strategic model of national rabies control in Korea. Clin Exp Vaccine Res. 2014; 3:78–90.
Article6). Sugiyama M, Yoshiki R, Tatsuno Y, Hiraga S, Itoh O, Gamoh K, et al. A new competitive enzyme-linked immunosorbent assay demonstrates adequate immune levels to rabies virus in compulsorily vaccinated Japanese domestic dogs. Clin Diagn Lab Immunol. 1997; 4:727–30.
Article7). He Y, Gao D, Zhang M. Expression of the nucleoprotein gene of rabies virus for use as a diagnostic reagent. J Virol Methods. 2006; 138:147–51.
Article8). Knoop EV, Freuling CM, Kliemt J, Selhorst T, Conraths FJ, Müller T. Evaluation of a commercial rabies ELISA as a replacement for serum neutralization assays as part of the pet travel scheme and oral vaccination campaigns of foxes. Berl Munch Tierarztl Wochenschr. 2010; 123:278–85.9). Wasniewski M, Almeida I, Baur A, Bedekovic T, Boncea D, Chaves LB, et al. First international collaborative study to evaluate rabies antibody detection method for use in monitoring the effectiveness of oral vaccination programmes in fox and raccoon dog in Europe. J Virol Methods. 2016; 238:77–85.
Article10). Franka R, Smith TG, Dyer JL, Wu X, Niezgoda M. Current and future tools for global canine rabies elimination. Antiviral Res. 2013; 100:220–5.
Article11). Inoue S, Motoi Y, Kashimura T, Ono K, Yamada A. Safe and easy monitoring of anti-rabies antibody in dogs using his-tagged recombinant N-protein. Jpn J Infect Dis. 2003; 56:158–60.12). Tursunov K, Begaliyeva A, Ingirbay B, Mukanov K, Ramanculov E, Shustov A, et al. Cloning and expression of fragment of the rabies virus nucleoprotein gene in Escherichia coli and evaluation of antigenicity of the expression product. Iran J Vet Res. 2017; 18:36–42.13). Reid-Sanden FL, Sumner JW, Smith JS, Fekadu M, Shaddock JH, Bellini WJ. Rabies diagnostic reagents prepared from a rabies N gene recombinant expressed in baculovirus. J Clin Microbiol. 1990; 28:858–63.
Article14). Kucinskaite I, Juozapaitis M, serva A, Zvirbliene A, Johnson N, Staniulis J, et al. Antigenic characterization of yeast-expressed lyssavirus nucleoproteins. Virus Genes. 2007; 35:521–9.15). Perea Arango I, Loza Rubio E, Rojas Anaya E, Olivera Flores T, Gonzalez de la Vara L, Gómez Lim MA. Expression of the rabies virus nucleoprotein in plants at high levels and evaluation of immune responses in mice. Plant Cell Rep. 2008; 27:677–85.16). Schrijver RS, Kramps JA. Critical factors affecting the diagnostic reliability of enzyme-linked immunosorbent assay formats. Rev Sci Tech. 1998; 17:550–61.17). Zeng N, Gong M, Guo L, Qiu W, Li G. Development of SPA-ELISA for detection of antibodies against rabies virus based on expression of main antigenic determinant of nucleoprotein. Sheng Wu Gong Cheng Xue Bao. 2011; 27:1149–57.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Indirect ELISA for the Detection of Rabies Virus Antibodies in Dog Sera
- Seroprevalence of rabies virus antibody for dogs and cats in Seoul during 2017–2019
- Development of an Indirect ELISA Featuring Plates Coated with Column Chromatographically Purified Canine Adenovirus Type-1 Antigen
- Development of indirect ELISA for the detection of canine adenovirus type 2 antibodies in dog sera
- Detection of immunity in sheep following anti-rabies vaccination