J Bacteriol Virol.
2012 Mar;42(1):69-75. 10.4167/jbv.2012.42.1.69.
Enhancement of Protein Productivity of Recombinant Hepatitis A Virus VP1 in Stably Transfected Drosophila melanogaster S2 Cells
- Affiliations
-
- 1Graduate School of Biotechnology, Kyung Hee University, Yongin, Korea. ischung@khu.ac.kr
- 2Medican Co., Ltd., Seoul, Korea.
- 3Korea Food Research Institute, Seongnam-si, Kyunggi-do, Korea.
- 4Department of Microbiology & Research Institute for Translational System Biomics, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 1434775
- DOI: http://doi.org/10.4167/jbv.2012.42.1.69
Abstract
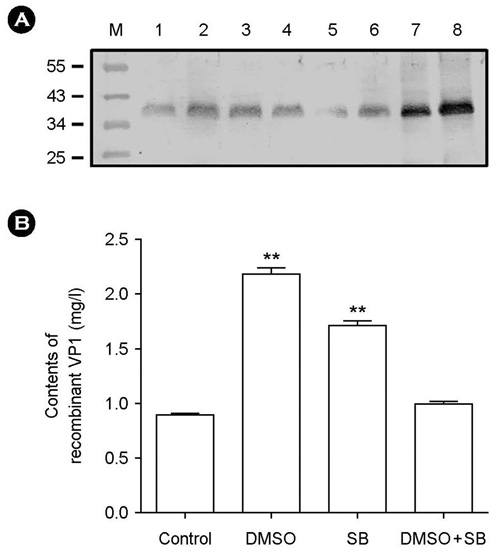
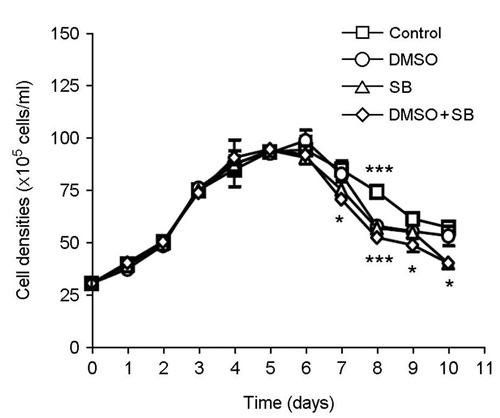
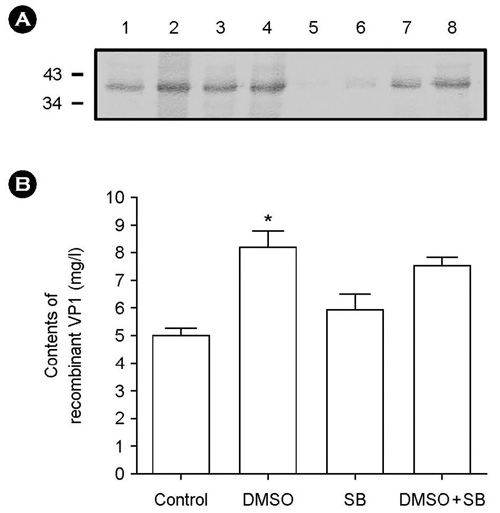
- The effect of DMSO and sodium butyrate on the production of recombinant hepatitis A virus (HAV) capsid protein VP1 was evaluated and optimized in the culture of stably transfected Drosophila melanogaster S2 cells using culture plates and spinner flasks. The effect of DMSO and sodium butyrate was also evaluated to improve the recombinant VP1 production in stably transfected Drosophila S2 cells. A production level of 0.88 mg of recombinant VP1/liter was obtained in the culture-plate culture of stably transfected S2 cells at 6 days after induction with 0.5 mM CuSO4. The supplements of 2% DMSO and 10 mM sodium butyrate at 4 days post-inoculation increased recombinant VP1 accumulation by 141 and 104%, respectively, resulting in 2.17 and 1.7 mg/liter of recombinant VP1 production. In spinner flasks, recombinant VP1 production reached maximum level at 9 days after induction with 0.5 mM CuSO4, with approximately 4.96 mg/liter of recombinant VP1 production level. When 2% DMSO or 10 mM sodium butyrate was added at 5 days post-inoculation, the recombinant VP1 production was increased to 8.35 and 5.85 mg/liter, respectively. However, the synergistic effects of DMSO and sodium butyrate were not observed. These results indicate that DMSO and/or sodium butyrate can be successfully used to improve the recombinant HAV VP1 production in culture plates and spinner flasks.
Keyword
MeSH Terms
Figure
Reference
-
1. Hughes JV, Stanton LW, Tomassini JE, Long WJ, Scolnick EM. Neutralizing monoclonal antibodies to hepatitis A virus: partial localization of a neutralizing antigenic site. J Virol. 1984. 52:465–473.
Article2. Hughes JV, Stanton LW. Isolation and immunizations with hepatitis A viral structural proteins: induction of antiprotein, antiviral, and neutralizing responses. J Virol. 1985. 55:395–401.
Article3. Stapleton JT, Lemon SM. Neutralization escape mutants define a dominant immunogenic neutralization site on hepatitis A virus. J Virol. 1987. 61:491–498.
Article4. Emini EA, Hughes JV, Perlow DS, Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985. 55:836–839.
Article5. Arnon R. Synthetic peptides as the basis for vaccine design. Mol Immunol. 1991. 28:209–215.
Article6. Ostermayr R, von der Helm K, Gauss-Müller V, Winnacker EL, Deinhardt F. Expression of hepatitis A virus cDNA in Escherichia coli: antigenic VP1 recombinant proteins. J Virol. 1987. 61:3645–3647.
Article7. Johnston JM, Harmon SA, Binn LN, Richards OC, Ehrenfeld E, Summers DF. Antigenic and immunogenic properties of a hepatitis A virus capsid protein expressed in Escherichia coli. J Infect Dis. 1988. 157:1203–1211.
Article8. Gauss-Muller V, Zhou MQ, von der Helm K, Deinhardt F. Recombinant proteins VP1 and VP3 of hepatitis A virus prime for neutralizing response. J Med Virol. 1990. 31:277–283.
Article9. Harmon SA, Johnston JM, Ziegelhoffer T, Richards OC, Summers DF, Ehrenfeld E. Expression of hepatitis A virus capsid sequences in insect cells. Virus Res. 1988. 10:273–280.
Article10. Lee JM, Lee HH, Hwang-Bo J, Shon DH, Kim W, Chung IS. Expression and immunogenicity of recombinant polypeptide VP1 of human hepatitis A virus in stably transformed fruitfly (Drosophila melanogaster) Schneider 2 cells. Biotechnol Appl Biochem. 2009. 53:101–109.
Article11. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970. 227:680–685.
Article12. Schmidt AJ, Lee JM, An G. Media and environmental effects on phenolics production from Tobacco cell cultures. Biotechnol Bioeng. 1989. 33:1437–1444.
Article13. Wahl MF, An GH, Lee JM. Effects of dimethyl sulfoxide on heavy chain monoclonal antibody production from plant cell culture. Biotechnol Lett. 1995. 17:463–468.
Article14. Rodriguez J, Spearman M, Huzel N, Butler M. Enhanced production of monomeric interferon-beta by CHO cells through the control of culture conditions. Biotechnol Prog. 2005. 21:22–30.
Article15. Li J, Sun X, Zhang Y. Improvement of hepatitis B surface antigen expression by dimethyl sulfoxide in the culture of recombinant Chinese hamster ovary cells. Process Biochem. 2006. 41:317–322.
Article16. Palermo DP, DeGraaf ME, Marotti KR, Rehberg E, Post LE. Production of analytical quantities of recombinant proteins in Chinese hamster ovary cells using sodium butyrate to elevate gene expression. J Biotechnol. 1991. 19:35–47.
Article17. Oster T, Thioudellet C, Chevalot I, Masson C, Wellman M, Marc A, et al. Induction of recombinant human gamma-glutamyl transferase by sodium butyrate in transfected V79 and CHO Chinese hamster cells. Biochem Biophys Res Commun. 1993. 193:406–412.
Article18. Chang KH, Park JH, Lee YH, Kim JH, Chun HO, Kim JH, et al. Dimethylsulfoxide and sodium butyrate enhance the production of recombinant cyclooxygenase 2 in stably transformed Drosophila melanogaster S2 cells. Biotechnol Lett. 2002. 24:1353–1359.19. Oh SK, Vig P, Chua F, Teo WK, Yap MG. Substantial overproduction of antibodies by applying osmotic pressure and sodium butyrate. Biotechnol Bioeng. 1993. 42:601–610.
Article20. Arts J, Lansink M, Grimbergen J, Toet KH, Kooistra T. Stimulation of tissue-type plasminogen activator gene expression by sodium butyrate and trichostatin A in human endothelial cells involves histone acetylation. Biochem J. 1995. 310:171–176.
Article21. Park JH, Chang KH, Lee YH, Kim HY, Yang JM, Chung IS. Production of recombinant rotavirus capsid protein VP7 from stably transformed Drosophila melanogaster S2 cells. J Microbial Biotechnol. 2002. 12:563–568.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhancement of Protein Productivity of Recombinant Hepatitis A Virus VP1 in Stably Transfected Drosophila melanogaster S2 Cells
- Recombination and Expression of VP1 Gene of Infectious Pancreatic Necrosis Virus DRT Strain in a Baculovirus,Hyphantria cunea Nuclear Polyhedrosis Virus
- Activation of B cells using Schneider 2 cells expressing CD40 ligand for the enhancement of antigen presentation in vitro
- Drosophila melanogaster as a Model for Studying Aspergillus fumigatus
- Expression of human norovirus VP1 gene and VP1-specific monoclonal antibodies