Cell-based Immunotherapy for Colorectal Cancer with Cytokine-induced Killer Cells
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju 28644, Korea. shan@chungbuk.ac.kr
- KMID: 2168050
- DOI: http://doi.org/10.4110/in.2016.16.2.99
Abstract
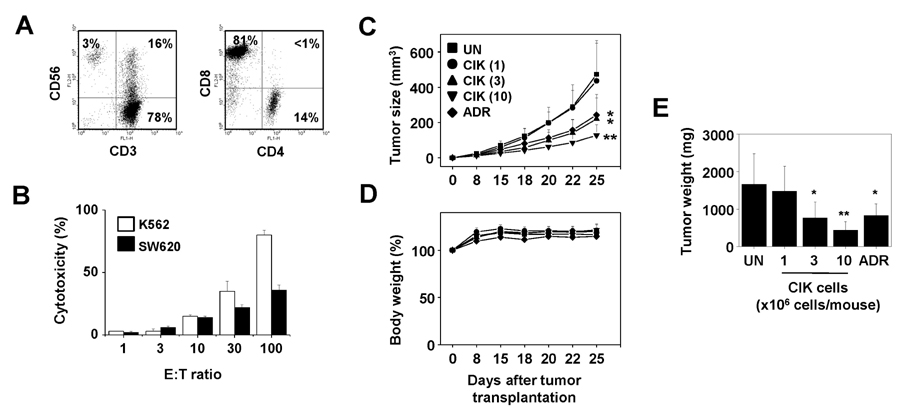
- Colorectal cancer is the third leading cancer worldwide. Although incidence and mortality of colorectal cancer are gradually decreasing in the US, patients with metastatic colorectal cancer have poor prognosis with an estimated 5-year survival rate of less than 10%. Over the past decade, advances in combination chemotherapy regimens for colorectal cancer have led to significant improvement in progression-free and overall survival. However, patients with metastatic disease gain little clinical benefit from conventional therapy, which is associated with grade 3~4 toxicity with negative effects on quality of life. In previous clinical studies, cell-based immunotherapy using dendritic cell vaccines and sentinel lymph node T cell therapy showed promising therapeutic results for metastatic colorectal cancer. In our preclinical and previous clinical studies, cytokine-induced killer (CIK) cells treatment for colorectal cancer showed favorable responses without toxicities. Here, we review current treatment options for colorectal cancer and summarize available clinical studies utilizing cell-based immunotherapy. Based on these studies, we recommend the use CIK cell therapy as a promising therapeutic strategy for patients with metastatic colorectal cancer.
MeSH Terms
Figure
Cited by 5 articles
-
Cytokine Signaling in Tumor Progression
Myungmi Lee, Inmoo Rhee
Immune Netw. 2017;17(4):214-227. doi: 10.4110/in.2017.17.4.214.Far Beyond Cancer Immunotherapy: Reversion of Multi-Malignant Phenotypes of Immunotherapeutic-Resistant Cancer by Targeting the NANOG Signaling Axis
Se Jin Oh, Jaeyoon Lee, Yukang Kim, Kwon-Ho Song, Eunho Cho, Minsung Kim, Heejae Jung, Tae Woo Kim
Immune Netw. 2020;20(1):e7. doi: 10.4110/in.2020.20.e7.Heterogeneity of Human γδ T Cells and Their Role in Cancer Immunity
Hye Won Lee, Yun Shin Chung, Tae Jin Kim
Immune Netw. 2020;20(1):e5. doi: 10.4110/in.2020.20.e5.Heterogeneity of Human γδ T Cells and Their Role in Cancer Immunity
Hye Won Lee, Yun Shin Chung, Tae Jin Kim
Immune Netw. 2020;20(1):. doi: 10.4110/in.2020.20.e5.Far Beyond Cancer Immunotherapy: Reversion of Multi-Malignant Phenotypes of Immunotherapeutic-Resistant Cancer by Targeting the NANOG Signaling Axis
Se Jin Oh, Jaeyoon Lee, Yukang Kim, Kwon-Ho Song, Eunho Cho, Minsung Kim, Tae Woo Kim, Heejae Jung
Immune Netw. 2020;20(1):. doi: 10.4110/in.2020.20.e7.
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.
Article2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29.
Article3. Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009; 18:1688–1694.
Article4. Kindler HL, Shulman KL. Metastatic colorectal cancer. Curr Treat Options Oncol. 2001; 2:459–471.
Article5. Sanoff HK, Sargent DJ, Campbell ME, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Goldberg RM. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008; 26:5721–5727.
Article6. Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Holmberg L, Kim DH, Malila N, Miller AB, Pietinen P, Rohan TE, Sellers TA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004; 140:603–613.7. Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009; 124:2406–2415.
Article8. Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Norat T. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011; 6:e20456.
Article9. Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, Qin H. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013; 8:e53916.
Article10. Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011; 26:863–876.
Article11. Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012; 10:639–645.
Article12. Taylor DP, Burt RW, Williams MS, Haug PJ, Cannon-Albright LA. Population-based family history-specific risks for colorectal cancer: a constellation approach. Gastroenterology. 2010; 138:877–885.
Article13. Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009; 22:191–197.
Article14. von Roon AC, Reese G, Teare J, Constantinides V, Darzi AW, Tekkis PP. The risk of cancer in patients with Crohn's disease. Dis Colon Rectum. 2007; 50:839–855.
Article15. Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008; 14:3937–3947.
Article16. Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Clendenning M, Sotamaa K, Prior T, Westman JA, Panescu J, Fix D, Lockman J, LaJeunesse J, Comeras I, de la Chapelle A. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008; 26:5783–5788.
Article17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010; 138:2044–2058.
Article18. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990; 61:759–767.
Article19. Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998; 396:643–649.
Article20. Lothe RA, Peltomaki P, Meling GI, Aaltonen LA, Nystrom-Lahti M, Pylkkanen L, Heimdal K, Andersen TI, Moller P, Rognum TO, Fossa SD, Haldorsen T, Langmark F, Brogger A, de la Chapelle A, Borresen A. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993; 53:5849–5852.21. Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008; 135:1079–1099.
Article22. Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992; 359:235–237.
Article23. Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin JP, Jarvinen H, Powell SM, Jen J, Hamilton SR, Petersen GM, Kinzler KW, Vogelstein B, de la Chapellet A. Clues to the pathogenesis of familial colorectal cancer. Science. 1993; 260:812–816.
Article24. Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993; 363:558–561.
Article25. Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, Jessup JM, Kolodner R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997; 57:808–811.26. Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010; 375:1030–1047.
Article27. Shen L, Toyota M, Kondo Y, Lin E, Zhang L, Guo Y, Hernandez NS, Chen X, Ahmed S, Konishi K, Hamilton SR, Issa JP. Integrated genetic and epigenetic analysis identifies three different subclasses of colon cancer. Proc Natl Acad Sci U S A. 2007; 104:18654–18659.
Article28. Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. 2010; 138:2059–2072.
Article29. Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E, Brouwers M, Charette M, Haller DG. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004; 22:3408–3419.
Article30. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014; 383:1490–1502.
Article31. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995; 345:939–944.32. Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004; 22:229–237.
Article33. Andrea C, Fausto P, Francesca BK, Mary C, Mara G, Veronica L, Sandro B. Which strategy after first-line therapy in advanced colorectal cancer? World J Gastroenterol. 2014; 20:8921–8927.34. Braun MS, Seymour MT. Balancing the efficacy and toxicity of chemotherapy in colorectal cancer. Ther Adv Med Oncol. 2011; 3:43–52.
Article35. Hohla F, Winder T, Greil R, Rick FG, Block NL, Schally AV. Targeted therapy in advanced metastatic colorectal cancer: current concepts and perspectives. World J Gastroenterol. 2014; 20:6102–6112.
Article36. Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015; 33:1809–1824.
Article37. Ahmed S, Johnson K, Ahmed O, Iqbal N. Advances in the management of colorectal cancer: from biology to treatment. Int J Colorectal Dis. 2014; 29:1031–1042.
Article38. Ueno H, Schmitt N, Klechevsky E, Pedroza-Gonzalez A, Matsui T, Zurawski G, Oh S, Fay J, Pascual V, Banchereau J, Palucka K. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010; 234:199–212.
Article39. Obeid J, Hu Y, Slingluff CL Jr. Vaccines, adjuvants, and dendritic cell activators--current status and future challenges. Semin Oncol. 2015; 42:549–561.
Article40. Liu KJ, Wang CC, Chen LT, Cheng AL, Lin DT, Wu YC, Yu WL, Hung YM, Yang HY, Juang SH, Whang-Peng J. Generation of carcinoembryonic antigen (CEA)-specific T-cell responses in HLA-A*0201 and HLA-A*2402 late-stage colorectal cancer patients after vaccination with dendritic cells loaded with CEA peptides. Clin Cancer Res. 2004; 10:2645–2651.
Article41. Sakakibara M, Kanto T, Hayakawa M, Kuroda S, Miyatake H, Itose I, Miyazaki M, Kakita N, Higashitani K, Matsubara T, Hiramatsu N, Kasahara A, Takehara T, Hayashi N. Comprehensive immunological analyses of colorectal cancer patients in the phase I/II study of quickly matured dendritic cell vaccine pulsed with carcinoembryonic antigen peptide. Cancer Immunol Immunother. 2011; 60:1565–1575.
Article42. Toh HC, Wang WW, Chia WK, Kvistborg P, Sun L, Teo K, Phoon YP, Soe Y, Tan SH, Hee SW, Foo KF, Ong S, Koo WH, Zocca MB, Claesson MH. Clinical benefit of allogeneic melanoma cell lysate-pulsed autologous dendritic cell vaccine in MAGE-positive colorectal cancer patients. Clin Cancer Res. 2009; 15:7726–7736.
Article43. Burgdorf SK, Fischer A, Myschetzky PS, Munksgaard SB, Zocca MB, Claesson MH, Rosenberg J. Clinical responses in patients with advanced colorectal cancer to a dendritic cell based vaccine. Oncol Rep. 2008; 20:1305–1311.
Article44. Hunyadi J, Andras C, Szabo I, Szanto J, Szluha K, Sipka S, Kovacs P, Kiss A, Szegedi G, Altorjay I, Sapy P, Antal-Szalmas P, Toth L, Fazekas G, Rajnavolgyi E. Autologous dendritic cell based adoptive immunotherapy of patients with colorectal cancer-A phase I-II study. Pathol Oncol Res. 2014; 20:357–365.
Article45. Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991; 5:344–366.
Article46. Kavanagh B, Ko A, Venook A, Margolin K, Zeh H, Lotze M, Schillinger B, Liu W, Lu Y, Mitsky P, Schilling M, Bercovici N, Loudovaris M, Guillermo R, Lee SM, Bender J, Mills B, Fong L. Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J Immunother. 2007; 30:762–772.
Article47. Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwartzentruber D, Wei JP, White DE. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988; 319:1676–1680.
Article48. Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry RM, Marincola FM, Leitman SF, Seipp CA, Rogers-Freezer L, Morton KE, Nahvi A, Mavroukakis SA, White DE, Rosenberg SA. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002; 25:243–251.
Article49. Gardini A, Ercolani G, Riccobon A, Ravaioli M, Ridolfi L, Flamini E, Ridolfi R, Grazi GL, Cavallari A, Amadori D. Adjuvant, adoptive immunotherapy with tumor infiltrating lymphocytes plus interleukin-2 after radical hepatic resection for colorectal liver metastases: 5-year analysis. J Surg Oncol. 2004; 87:46–52.
Article50. Karlsson M, Marits P, Dahl K, Dagoo T, Enerback S, Thorn M, Winqvist O. Pilot study of sentinel-node-based adoptive immunotherapy in advanced colorectal cancer. Ann Surg Oncol. 2010; 17:1747–1757.
Article51. Zhen YH, Liu XH, Yang Y, Li B, Tang JL, Zeng QX, Hu J, Zeng XN, Zhang L, Wang ZJ, Li XY, Ge HX, Winqvist O, Hu PS, Xiu J. Phase I/II study of adjuvant immunotherapy with sentinel lymph node T lymphocytes in patients with colorectal cancer. Cancer Immunol Immunother. 2015; 64:1083–1093.
Article52. Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011; 19:620–626.
Article53. Schmidt-Wolf GD, Negrin RS, Schmidt-Wolf IG. Activated T cells and cytokine-induced CD3+CD56+ killer cells. Ann Hematol. 1997; 74:51–56.54. Franceschetti M, Pievani A, Borleri G, Vago L, Fleischhauer K, Golay J, Introna M. Cytokine-induced killer cells are terminally differentiated activated CD8 cytotoxic T-EMRA lymphocytes. Exp Hematol. 2009; 37:616–628.e612.55. Verneris MR, Baker J, Edinger M, Negrin RS. Studies of ex vivo activated and expanded CD8+ NK-T cells in humans and mice. J Clin Immunol. 2002; 22:131–136.56. Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, Introna M. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011; 118:3301–3310.
Article57. Jakel CE, Schmidt-Wolf IG. An update on new adoptive immunotherapy strategies for solid tumors with cytokine-induced killer cells. Expert Opin Biol Ther. 2014; 14:905–916.
Article58. Zhu Y, Zhang H, Li Y, Bai J, Liu L, Liu Y, Qu Y, Qu X. Efficacy of postoperative adjuvant transfusion of cytokine-induced killer cells combined with chemotherapy in patients with colorectal cancer. Cancer Immunol Immunother. 2013; 62:1629–1635.
Article59. Zhu H, Yang X, Li J, Ren Y, Zhang T, Zhang C, Zhang J, Li J, Pang Y. Immune response, safety, and survival and quality of life outcomes for advanced colorectal cancer patients treated with dendritic cell vaccine and cytokine-induced killer cell therapy. Biomed Res Int. 2014; 2014:603871.
Article60. Gao D, Li C, Xie X, Zhao P, Wei X, Sun W, Liu HC, Alexandrou AT, Jones J, Zhao R, Li JJ. Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients. PLoS One. 2014; 9:e93886.
Article61. Leibovitz A, Stinson JC, McCombs WB 3rd, McCoy CE, Mazur KC, Mabry ND. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976; 36:4562–4569.62. Ramirez R, Solana R, Carracedo J, Alonso MC, Pena J. Mechanisms involved in NK resistance induced by interferon-gamma. Cell Immunol. 1992; 140:248–256.63. Esin E, Yalcin S. Maintenance strategy in metastatic colorectal cancer: A systematic review. Cancer Treat Rev. 2016; 42:82–90.
Article64. Ko JJ, Kennecke HF, Lim HJ, Renouf DJ, Gill S, Woods R, Speers C, Cheung WY. Reasons for underuse of adjuvant chemotherapy in elderly patients with stage III colon cancer. Clin Colorectal Cancer. 2015; DOI: 10.1016/j.clcc.2015.09.002.
Article65. Morse MA, Niedzwiecki D, Marshall JL, Garrett C, Chang DZ, Aklilu M, Crocenzi TS, Cole DJ, Dessureault S, Hobeika AC, Osada T, Onaitis M, Clary BM, Hsu D, Devi GR, Bulusu A, Annechiarico RP, Chadaram V, Clay TM, Lyerly HK. A randomized phase II study of immunization with dendritic cells modified with poxvectors encoding CEA and MUC1 compared with the same poxvectors plus GM-CSF for resected metastatic colorectal cancer. Ann Surg. 2013; 258:879–886.
Article66. Morse MA, Clay TM, Hobeika AC, Osada T, Khan S, Chui S, Niedzwiecki D, Panicali D, Schlom J, Lyerly HK. Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clin Cancer Res. 2005; 11:3017–3024.
Article67. Matsuda K, Tsunoda T, Tanaka H, Umano Y, Tanimura H, Nukaya I, Takesako K, Yamaue H. Enhancement of cytotoxic T-lymphocyte responses in patients with gastrointestinal malignancies following vaccination with CEA peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2004; 53:609–616.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cell-based Immunotherapy for Colorectal Cancer with Cytokine-induced Killer Cells
- Two Cases of Adjuvant Immunotherapy with Cytokine-Induced Killer Cells for Relapsed or Refractory Neuroblastoma
- Inhibition of Human Pancreatic Tumor Growth by Cytokine-Induced Killer Cells in Nude Mouse Xenograft Model
- A Case of Management for Advanced Hepatocellular Carcinoma with Extrahepatic Metastasis by Autologous Natural Killer Cells Combined with Immune Checkpoint Inhibitor
- Recent Advances and Future Directions in Immunotherapeutics for Hepatocellular Carcinoma