Immune Netw.
2013 Feb;13(1):10-15. 10.4110/in.2013.13.1.10.
Alum Directly Modulates Murine B Lymphocytes to Produce IgG1 Isotype
- Affiliations
-
- 1Department of Molecular Bioscience, College of Biomedical Science, Kangwon National University, Chuncheon 200-701, Korea. phkim@kangwon.ac.kr
- KMID: 2150765
- DOI: http://doi.org/10.4110/in.2013.13.1.10
Abstract
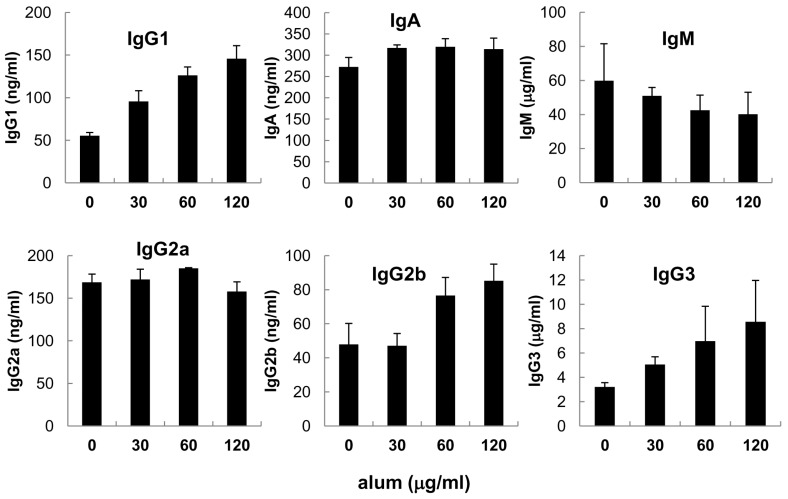
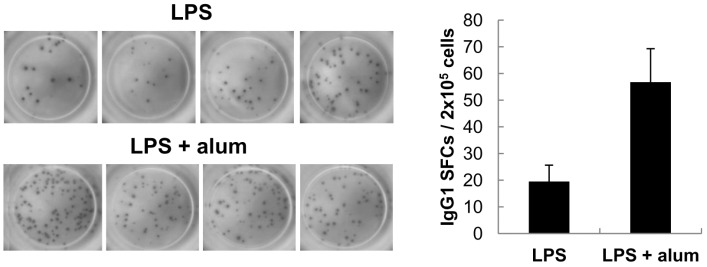
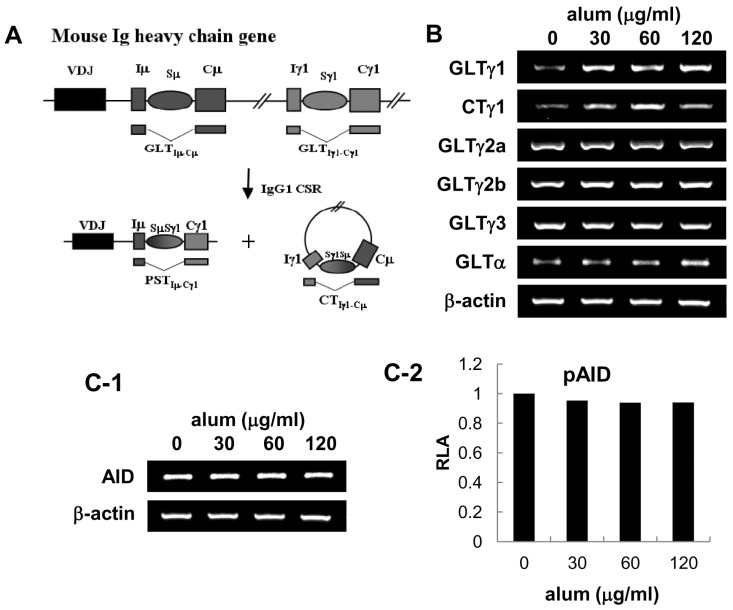
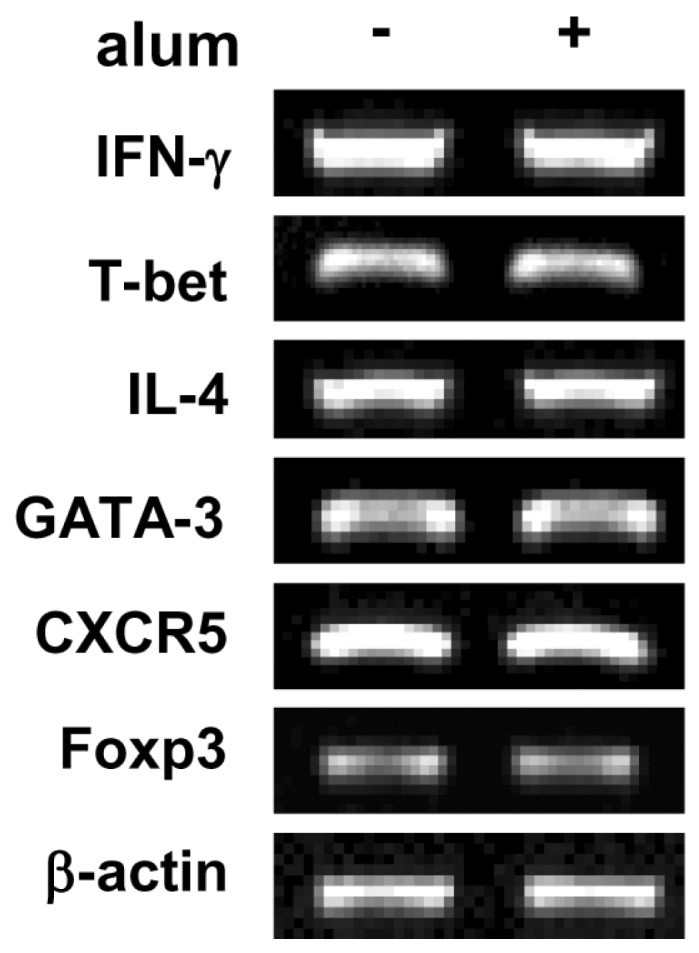
- Aluminum hydroxide (alum) is the most widely used adjuvant in human vaccines. Nevertheless, it is virtually unknown whether alum acts on B cells. In the present study, we explored the direct effect of alum on Ig expression by murine B cells in vitro. LPS-activated mouse spleen B cells were cultured with alum, and the level of isotype-specific Ig secretion, IgG1 secreting cell numbers, and Ig germ-line transcripts (GLT) were measured using ELISA, ELISPOT, and RT-PCR, respectively. Alum consistently enhanced total IgG1 production, numbers of IgG1 secreting cells, and GLTgamma1 expression. These results demonstrate that alum can directly cause IgG1 isotype switching leading to IgG1 production.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Nuclear Orphan Receptor NR4A1 is Involved in the Apoptotic Pathway Induced by LPS and Simvastatin in RAW 264.7 Macrophages
Yong Chan Kim, Seok Bean Song, Sang Kyu Lee, Sang Min Park, Young Sang Kim
Immune Netw. 2014;14(2):116-122. doi: 10.4110/in.2014.14.2.116.
Reference
-
1. Glenny A, Pope C, Waddington H, Wallace U. The antigenic value of toxoid precipitated by potassium alum. J Pathol Bacteriol. 1926; 26:38–39.2. Hutchison S, Benson RA, Gibson VB, Pollock AH, Garside P, Brewer JM. Antigen depot is not required for alum adjuvanticity. FASEB J. 2012; 26:1272–1279. PMID: 22106367.
Article3. Munks MW, McKee AS, Macleod MK, Powell RL, Degen JL, Reisdorph NA, Kappler JW, Marrack P. Aluminum adjuvants elicit fibrin-dependent extracellular traps in vivo. Blood. 2010; 116:5191–5199. PMID: 20876456.
Article4. Kool M, Soullié T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008; 205:869–882. PMID: 18362170.
Article5. Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008; 181:3755–3759. PMID: 18768827.
Article6. Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008; 181:17–21. PMID: 18566365.
Article7. Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008; 453:1122–1126. PMID: 18496530.
Article8. Franchi L, Núñez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008; 38:2085–2089. PMID: 18624356.9. McKee AS, Munks MW, MacLeod MK, Fleenor CJ, Van Rooijen N, Kappler JW, Marrack P. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009; 183:4403–4414. PMID: 19734227.
Article10. Allen AC, Layward L, Harper SJ, Feehally J. In vitro immunoglobulin isotype suppression in immunoglobulin A nephropathy. Exp Nephrol. 1994; 2:166–170. PMID: 7922268.11. Wang HB, Weller PF. Pivotal advance: eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J Leukoc Biol. 2008; 83:817–821. PMID: 18192490.
Article12. Shah HB, Devera TS, Rampuria P, Lang GA, Lang ML. Type II NKT cells facilitate Alum-sensing and humoral immunity. J Leukoc Biol. 2012; 92:883–893. PMID: 22798686.
Article13. Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005; 17:230–236. PMID: 15886111.
Article14. Nakamura M, Kondo S, Sugai M, Nazarea M, Imamura S, Honjo T. High frequency class switching of an IgM+ B lymphoma clone CH12F3 to IgA+ cells. Int Immunol. 1996; 8:193–201. PMID: 8671604.15. Park SR, Lee JH, Kim PH. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur J Immunol. 2001; 31:1706–1715. PMID: 11385614.16. Gonda H, Sugai M, Nambu Y, Katakai T, Agata Y, Mori KJ, Yokota Y, Shimizu A. The balance between Pax5 and Id2 activities is the key to AID gene expression. J Exp Med. 2003; 198:1427–1437. PMID: 14581609.
Article17. Kim PH, Kagnoff MF. Transforming growth factor beta 1 increases IgA isotype switching at the clonal level. J Immunol. 1990; 145:3773–3778. PMID: 2246513.18. Bergstedt-Lindqvist S, Moon HB, Persson U, Möller G, Heusser C, Severinson E. Interleukin 4 instructs uncommitted B lymphocytes to switch to IgG1 and IgE. Eur J Immunol. 1988; 18:1073–1077. PMID: 3261245.19. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000; 102:553–563. PMID: 11007474.
Article20. Li SC, Rothman PB, Zhang J, Chan C, Hirsh D, Alt FW. Expression of I mu-C gamma hybrid germline transcripts subsequent to immunoglobulin heavy chain class switching. Int Immunol. 1994; 6:491–497. PMID: 8018590.21. Kinoshita K, Harigai M, Fagarasan S, Muramatsu M, Honjo T. A hallmark of active class switch recombination: transcripts directed by I promoters on looped-out circular DNAs. Proc Natl Acad Sci U S A. 2001; 98:12620–12623. PMID: 11606740.
Article22. Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell. 2000; 102:565–575. PMID: 11007475.
Article23. Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, Lekeux P, Coban C, Akira S, Ishii KJ, Bureau F, Desmet CJ. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011; 17:996–1002. PMID: 21765404.
Article24. Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, Ward SM, Seamone ME, Vilaysane A, Mucsi AD, Fong Y, Prenner E, Ling CC, Tschopp J, Muruve DA, Amrein MW, Shi Y. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med. 2011; 17:479–487. PMID: 21399646.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alum Directly Modulates Murine B Lymphocytes to Produce IgG1 Isotype
- Newly Identified TLR9 Stimulant, M6-395 Is a Potent Polyclonal Activator for Murine B Cells
- Vitamin C acts indirectly to modulate isotype switching in mouse B cells
- T Cell Dependent Antigen-Induced Immunoglobulin Isotype Swiching and Diifferentiation of Lymph Node
- Role of murine Peyer's patch lymphocytes against primary and challenge infections with Cryptosporidium parvum