Yonsei Med J.
2017 Jul;58(4):848-858. 10.3349/ymj.2017.58.4.848.
Efficacy of Palonosetron vs. Ramosetron for the Prevention of Postoperative Nausea and Vomiting: A Meta-Analysis of Randomized Controlled Trials
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Severance Hospital, Seoul, Korea. skshin@yuhs.ac
- 2Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2419094
- DOI: http://doi.org/10.3349/ymj.2017.58.4.848
Abstract
- PURPOSE
This study was designed as a meta-analysis of randomized controlled trials (RCTs) that included the comparison of palonosetron and ramosetron for postoperative nausea and vomiting (PONV) prophylaxis.
MATERIALS AND METHODS
A systematic search was conducted for the PubMed, EMBASE, Web of Science, CENTRAL, KoreaMed, and Google Scholar databases (PROSPERO protocol number CRD42015026009). Primary outcomes were the incidences of postoperative nausea (PON) and postoperative vomiting (POV) during the first 48 hrs after surgery. The total 48-hr period was further analyzed in time epochs of 0-6 hrs (early), 6-24 hrs (late), and 24-48 hrs (delayed). Subgroup analyses according to number of risk factors, sex, and type of surgery were also performed.
RESULTS
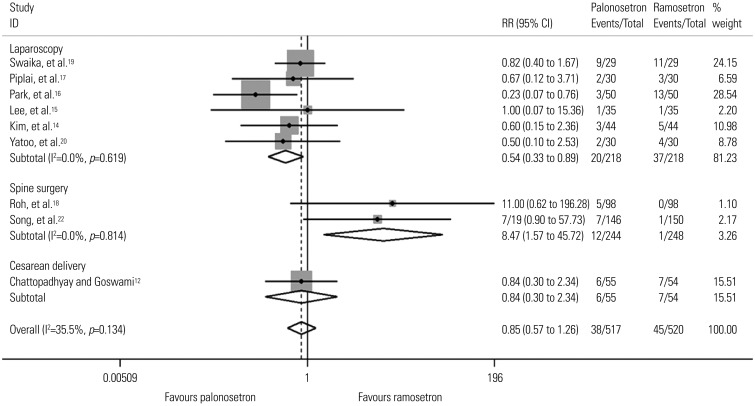
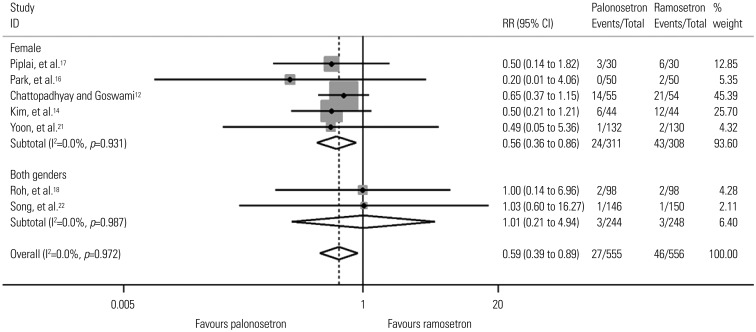
Eleven studies including 1373 patients were analyzed. There was no difference in PON or POV between the two drugs for the total 48-hr period after surgery. However, palonosetron was more effective in preventing POV during the delayed period overall [relative risk (RR), 0.59; 95% confidence interval (CI), 0.39 to 0.89; p=0.013], as well as after subgroup analyses for females and laparoscopies (RR, 0.56; 95% CI, 0.36 to 0.86; p=0.009 and RR, 0.46; 95% CI, 0.23 to 0.94; p=0.033). Subgroup analysis for spine surgery showed that ramosetron was more effective in reducing POV during the total 48-hr (RR, 3.34; 95% CI, 1.46 to 7.63; p=0.004) and early periods (RR, 8.47; 95% CI, 1.57 to 45.72; p=0.013).
CONCLUSION
This meta-analysis discovered no definite difference in PONV prevention between the two drugs. The significant findings that were seen in different time epochs and subgroup analyses should be confirmed in future RCTs.
MeSH Terms
-
Adult
Antiemetics/therapeutic use
Benzimidazoles/*therapeutic use
Female
Humans
Isoquinolines/*therapeutic use
Male
Middle Aged
Postoperative Nausea and Vomiting/chemically induced/*drug therapy/*prevention & control
Publication Bias
Quinuclidines/*therapeutic use
*Randomized Controlled Trials as Topic
Risk Factors
Treatment Outcome
Antiemetics
Benzimidazoles
Isoquinolines
Quinuclidines
Figure
Cited by 2 articles
-
Scheduled injection of ramosetron for prevention of nausea and vomiting following single-port access total laparoscopic hysterectomy: a prospective randomized study
Shoou-Chern Li, Youngmi Wang, Seong Jin Choi, Yeon Soo Jung, Kyoung-Hee Han, In Bai Chung, San-Hui Lee
Obstet Gynecol Sci. 2019;62(5):344-351. doi: 10.5468/ogs.2019.62.5.344.Introduction to systematic review and meta-analysis
EunJin Ahn, Hyun Kang
Korean J Anesthesiol. 2018;71(2):103-112. doi: 10.4097/kjae.2018.71.2.103.
Reference
-
1. Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014; 118:85–113. PMID: 24356162.2. Wiesmann T, Kranke P, Eberhart L. Postoperative nausea and vomiting-a narrative review of pathophysiology, pharmacotherapy and clinical management strategies. Expert Opin Pharmacother. 2015; 16:1069–1077. PMID: 25866213.3. Mihara T, Tojo K, Uchimoto K, Morita S, Goto T. Reevaluation of the effectiveness of ramosetron for preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Anesth Analg. 2013; 117:329–339. PMID: 23757469.4. Xiong C, Liu G, Ma R, Xue J, Wu A. Efficacy of palonosetron for preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Can J Anaesth. 2015; 62:1268–1278. PMID: 26296300.
Article5. Apfel CC, Philip BK, Cakmakkaya OS, Shilling A, Shi YY, Leslie JB, et al. Who is at risk for postdischarge nausea and vomiting after ambulatory surgery? Anesthesiology. 2012; 117:475–486. PMID: 22846680.
Article6. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009; 151:W65–W94. PMID: 19622512.
Article7. Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992; 77:162–184. PMID: 1609990.8. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005; 5:13. PMID: 15840177.
Article9. Maitra S, Baidya DK, Bhattacharjee S, Khanna P. Evaluation of igel(™) airway in children: a meta-analysis. Paediatr Anaesth. 2014; 24:1072–1079. PMID: 25041224.10. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. PMID: 22008217.
Article11. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174. PMID: 843571.
Article12. Chattopadhyay S, Goswami S. Palonosetron versus ramosetron prophylaxis for control of postoperative nausea and vomiting after cesarean delivery under spinal anesthesia. J Obstet Gynaecol India. 2015; 65:28–33. PMID: 25737619.
Article13. Kim SH, Hong JY, Kim WO, Kil HK, Karm MH, Hwang JH. Palonosetron has superior prophylactic antiemetic efficacy compared with ondansetron or ramosetron in high-risk patients undergoing laparoscopic surgery: a prospective, randomized, double-blinded study. Korean J Anesthesiol. 2013; 64:517–523. PMID: 23814652.
Article14. Kim SH, Oh CS, Lee SJ. Efficacy of palonosetron and ramosetron on postoperative nausea and vomiting related to intravenous patient-controlled analgesia with opioids after gynecological laparoscopic surgery (double-blinded prospective randomized controlled trial). J Anesth. 2015; 29:585–592. PMID: 25735497.
Article15. Lee WS, Lee KB, Lim S, Chang YG. Comparison of palonosetron, granisetron, and ramosetron for the prevention of postoperative nausea and vomiting after laparoscopic gynecologic surgery: a prospective randomized trial. BMC Anesthesiol. 2015; 15:121. PMID: 26335706.
Article16. Park SK, Cho EJ, Kang SH, Lee YJ, Kim DA. A randomized, doubleblind study to evaluate the efficacy of ramosetron and palonosetron for prevention of postoperative nausea and vomiting after gynecological laparoscopic surgery. Korean J Anesthesiol. 2013; 64:133–137. PMID: 23459596.
Article17. Piplai G, Chakrabarty I, Mukhopadhyay M, Karmakar M, Sarkar S, Bhattacharjee PD. A comparative study between palonosetron and ramosetron to prevent postoperative nausea and vomiting after laparoscopic cholecystectomy. Int Res J Pharm Pharmacol. 2012; 2:193–197.18. Roh GU, Yang SY, Shim JK, Kwak YL. Efficacy of palonosetron versus ramosetron on preventing opioid-based analgesia-related nausea and vomiting after lumbar spinal surgery: a prospective, randomized, and double-blind trial. Spine (Phila Pa 1976). 2014; 39:E543–E549. PMID: 24480956.19. Swaika S, Pal A, Chatterjee S, Saha D, Dawar N. Ondansetron, ramosetron, or palonosetron: which is a better choice of antiemetic to prevent postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy? Anesth Essays Res. 2011; 5:182–186. PMID: 25885385.
Article20. Yatoo FA, Mahotra KK, Mehta N, Gupta KC, Sidhra J. A comparative study of granisetron, ramosetron and palonosetron as antiemetics in prevention of postoperative nausea and vomiting in patients undergoing laparoscopic surgeries. J Evolution Med Dent Sci. 2016; 5:1229–1234.21. Yoon HJ, Jee YS, Kim YD. Comparison of the efficacy of ramosetron and palonosetron for prevention of postoperative nausea and vomiting in patients undergoing gynecologic oncology surgery. Anesth Pain Med. 2016; 11:264–268.
Article22. Song JW, Shim JK, Choi SH, Soh S, Jang J, Kwak YL. Comparison of ramosetron and palonosetron for preventing nausea and vomiting after spinal surgery: association with ABCB1 polymorphisms. J Neurosurg Anesthesiol. 2016; 8. 25. [Epub]. DOI: 10.1097/ANA.0000000000000361.
Article23. Gao C, Li B, Xu L, Lv F, Cao G, Wang H, et al. Efficacy and safety of ramosetron versus ondansetron for postoperative nausea and vomiting after general anesthesia: a meta-analysis of randomized clinical trials. Drug Des Devel Ther. 2015; 9:2343–2350.
Article24. Odom-Forren J, Jalota L, Moser DK, Lennie TA, Hall LA, Holtman J, et al. Incidence and predictors of postdischarge nausea and vomiting in a 7-day population. J Clin Anesth. 2013; 25:551–559. PMID: 23988801.
Article25. Rojas C, Li Y, Zhang J, Stathis M, Alt J, Thomas AG, et al. The antiemetic 5-HT3 receptor antagonist Palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther. 2010; 335:362–368. PMID: 20724484.
Article26. Rojas C, Thomas AG, Alt J, Stathis M, Zhang J, Rubenstein EB, et al. Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol. 2010; 626:193–199. PMID: 19836386.
Article27. Rojas C, Stathis M, Thomas AG, Massuda EB, Alt J, Zhang J, et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg. 2008; 107:469–478. PMID: 18633025.
Article28. Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000; 59:213–243. PMID: 10730546.
Article29. Yang LP, Scott LJ. Palonosetron: in the prevention of nausea and vomiting. Drugs. 2009; 69:2257–2278. PMID: 19852528.30. Kim SH, Lee SM, Kim YK, Park SY, Lee JH, Cho SH, et al. Effects of prophylactic ramosetron and ondansetron on corrected QT interval during general anesthesia. J Clin Anesth. 2014; 26:511–516. PMID: 25439413.
Article31. Kwon MJ, Han J, Seo JH, Song K, Jeong HM, Choi JS, et al. CD24 overexpression is associated with poor prognosis in luminal A and triple-negative breast cancer. PLoS One. 2015; 10:e0139112. PMID: 26444008.
Article32. Kim HJ, Lee HC, Jung YS, Lee J, Min JJ, Hong DM, et al. Effect of palonosetron on the QTc interval in patients undergoing sevoflurane anaesthesia. Br J Anaesth. 2014; 112:460–468. PMID: 24129597.
Article33. Min JJ, Kim HJ, Jung SY, Kim BG, Kwon K, Jung HJ, et al. Effects of palonosetron on perioperative cardiovascular complications in patients undergoing noncardiac surgery with general anesthesia: a retrospective cohort study. Clin Pharmacol Ther. 2015; 98:96–106. PMID: 25786663.
Article34. Rodseth RN, Gopalan PD, Cassimjee HM, Goga S. Reduced incidence of postoperative nausea and vomiting in black South Africans and its utility for a modified risk scoring system. Anesth Analg. 2010; 110:1591–1594. PMID: 20385613.
Article35. Stern RM, Hu S, Uijtdehaage SH, Muth ER, Xu LH, Koch KL. Asian hypersusceptibility to motion sickness. Hum Hered. 1996; 46:7–14. PMID: 8825456.
Article36. Myles PS, Chan MT, Kasza J, Paech MJ, Leslie K, Peyton PJ, et al. Severe nausea and vomiting in the rvaluation of nitrous oxide in the gas mixture for anesthesia II trial. Anesthesiology. 2016; 124:1032–1040. PMID: 26904965.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Palonosetron vs. Ramosetron for the Prevention of Postoperative Nausea and Vomiting: A Meta-Analysis of Randomized Controlled Trials
- Comparison of the efficacy of ramosetron and palonosetron for prevention of postoperative nausea and vomiting in patients undergoing gynecologic oncology surgery
- Ramosetron for the prevention of postoperative nausea and vomiting (PONV): a meta-analysis
- A randomized, double-blind study to evaluate the efficacy of ramosetron and palonosetron for prevention of postoperative nausea and vomiting after gynecological laparoscopic surgery
- Palonosetron has superior prophylactic antiemetic efficacy compared with ondansetron or ramosetron in high-risk patients undergoing laparoscopic surgery: a prospective, randomized, double-blinded study