Molecular Approach to Allergy Diagnosis and Therapy
- Affiliations
-
- 1Christian Doppler Laboratory for Allergy Diagnosis and Therapy, Department of Molecular Biology, University of Salzburg, Salzburg, Austria. fatima.ferreira@sbg.ac.at
- KMID: 2130806
- DOI: http://doi.org/10.3349/ymj.2014.55.4.839
Abstract
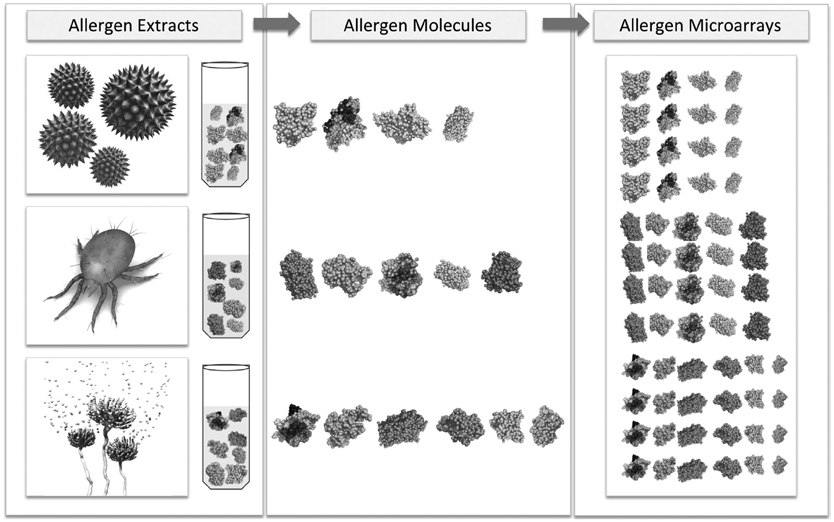
- Presently, allergy diagnosis and therapy procedures are undergoing a transition phase in which allergen extracts are being step-by-step replaced by molecule-based products. The new developments will allow clinicians to obtain detailed information on sensitization patterns, more accurate interpretation of allergic symptoms, and thus improved patients' management. In this respect, recombinant technology has been applied to develop this new generation of molecule-based allergy products. The use of recombinant allergens allows full validation of identity, quantity, homogeneity, structure, aggregation, solubility, stability, IgE-binding and the biologic potency of the products. In contrast, such parameters are extremely difficult to assay and standardize for extract-based products. In addition to the possibility of bulk production of wild type molecules for diagnostic purposes, recombinant technology opened the possibility of developing safer and more efficacious products for allergy therapy. A number of molecule-based hypoallergenic preparations have already been successfully evaluated in clinical trials, bringing forward the next generation of allergy vaccines. In this contribution, we review the latest developments in allergen characterization, molecule-based allergy diagnosis, and the application of recombinant allergens in therapeutic setups. A comprehensive overview of clinical trials using recombinant allergens as well as synthetic peptides is presented.
Keyword
MeSH Terms
Figure
Cited by 6 articles
-
Different Responses in Induction of Allergen Specific Immunoglobulin G4 and IgE-Blocking Factors for Three Mite Subcutaneous Immunotherapy Products
Kyung Hee Park, Sang Chul Lee, Young Woong Son, Kyoung Yong Jeong, Yoo Seob Shin, Jung U Shin, Da Woon Sim, Hye Jung Park, Jae-Hyun Lee, Kwang Hoon Lee, Jung-Won Park
Yonsei Med J. 2016;57(6):1427-1434. doi: 10.3349/ymj.2016.57.6.1427.Retrospective Analysis on the Effects of House Dust Mite Specific Immunotherapy for More Than 3 Years in Atopic Dermatitis
Jungsoo Lee, Hemin Lee, Seongmin Noh, Byung Gi Bae, Jung U Shin, Chang Ook Park, Kwang Hoon Lee
Yonsei Med J. 2016;57(2):393-398. doi: 10.3349/ymj.2016.57.2.393.Near-Road Exposure and Impact of Air Pollution on Allergic Diseases in Elementary School Children: A Cross-Sectional Study
Ho Hyun Kim, Chung Soo Lee, Seung Do Yu, Jung Sub Lee, Jun Young Chang, Jun Min Jeon, Hye Rim Son, Chan Jung Park, Dong Chun Shin, Young Wook Lim
Yonsei Med J. 2016;57(3):698-713. doi: 10.3349/ymj.2016.57.3.698.Validation of PROTIA™ Allergy-Q 64 Atopy® as a Specific IgE Measurement Assay for 10 Major Allergen Components
Sung Ryeol Kim, Kyung Hee Park, Jae-Hyun Lee, Bum Joon Kim, Jae Hwan Hwang, Kook Jin Lim, Jung-Won Park
Allergy Asthma Immunol Res. 2019;11(3):422-432. doi: 10.4168/aair.2019.11.3.422.Physical and biochemical characteristics of allergens
Kyoung Yong Jeong
Allergy Asthma Respir Dis. 2016;4(3):157-166. doi: 10.4168/aard.2016.4.3.157.Allergen standardization
Jung-Won Park, Kyoung Yong Jeong
Allergy Asthma Respir Dis. 2018;6(4):191-196. doi: 10.4168/aard.2018.6.4.191.
Reference
-
1. Kay AB. Allergy and allergic diseases. Second of two parts. N Engl J Med. 2001; 344:109–113.2. Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001; 344:30–37.3. Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004; 59:469–478.
Article4. Ferreira F, Briza P, Inführ D, Schmidt G, Wallner M, Wopfner N, et al. Modified recombinant allergens for safer immunotherapy. Inflamm Allergy Drug Targets. 2006; 5:5–14.
Article5. Valenta R, Niederberger V. Recombinant allergens for immunotherapy. J Allergy Clin Immunol. 2007; 119:826–830.
Article6. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007; 119:780–791.
Article7. Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006; 6:761–771.
Article8. Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005; 116:608–613.
Article9. Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004; 101:Suppl 2. 14677–14682.
Article10. Tulic MK, Fiset PO, Christodoulopoulos P, Vaillancourt P, Desrosiers M, Lavigne F, et al. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J Allergy Clin Immunol. 2004; 113:235–241.
Article11. Moingeon P, Batard T, Fadel R, Frati F, Sieber J, Van Overtvelt L. Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy. 2006; 61:151–165.
Article12. Weiss R, Hammerl P, Hartl A, Hochreiter R, Leitner WW, Scheiblhofer S, et al. Design of protective and therapeutic DNA vaccines for the treatment of allergic diseases. Curr Drug Targets Inflamm Allergy. 2005; 4:585–597.
Article13. Weiss R, Scheiblhofer S, Gabler M, Ferreira F, Leitner WW, Thalhamer J. Is genetic vaccination against allergy possible? Int Arch Allergy Immunol. 2006; 139:332–345.
Article14. van Ree R. CREATE Partnership. The CREATE project: EU support for the improvement of allergen standardization in Europe. Allergy. 2004; 59:571–574.
Article15. Akkerdaas JH, Wensing M, Knulst AC, Krebitz M, Breiteneder H, de Vries S, et al. How accurate and safe is the diagnosis of hazelnut allergy by means of commercial skin prick test reagents? Int Arch Allergy Immunol. 2003; 132:132–140.
Article16. Curin M, Reininger R, Swoboda I, Focke M, Valenta R, Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011; 154:258–263.
Article17. U.S. Food and Drug Administration. Standardized Allergenic Extracts. USA: U.S. Food and Drug Administration;2009. Available from: http://www.fda.gov/BiologicsBloodVaccines/Allergenics/ucm391514.htm.18. Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011; 52:393–400.
Article19. van Ree R, Chapman MD, Ferreira F, Vieths S, Bryan D, Cromwell O, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008; 63:310–326.
Article20. EDQM. EDQM Annual Report 2011. France: EDQM;2011. Available from: http://www.edqm.eu/en/EDQM-Downloads-527.html.21. Vieths S, Barber D, Chapman M, Costanzo A, Daas A, Fiebig H, et al. Establishment of recombinant major allergens Bet v 1 and Phl p 5a as Ph. Eur. reference standards and validation of ELISA methods for their measurement. Results from feasibility studies. Pharmeur Bio Sci Notes. 2012; 2012:118–134.22. Himly M, Nony E, Chabre H, Van Overtvelt L, Neubauer A, van Ree R, et al. Standardization of allergen products: 1. Detailed characterization of GMP-produced recombinant Bet v 1.0101 as biological reference preparation. Allergy. 2009; 64:1038–1045.
Article23. Canonica GW, Ansotegui IJ, Pawankar R, Schmid-Grendelmeier P, van Hage M, Baena-Cagnani CE, et al. A WAO-ARIA-GA2LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013; 6:17.
Article24. Westritschnig K, Horak F, Swoboda I, Balic N, Spitzauer S, Kundi M, et al. Different allergenic activity of grass pollen allergens revealed by skin testing. Eur J Clin Invest. 2008; 38:260–267.
Article25. Tripodi S, Frediani T, Lucarelli S, Macrì F, Pingitore G, Di Rienzo Businco A, et al. Molecular profiles of IgE to Phleum pratense in children with grass pollen allergy: implications for specific immunotherapy. J Allergy Clin Immunol. 2012; 129:834–839.
Article26. Gadermaier G, Hauser M, Ferreira F. Allergens of weed pollen: an overview on recombinant and natural molecules. Methods. 2014; 66:55–66.
Article27. Asero R, Wopfner N, Gruber P, Gadermaier G, Ferreira F. Artemisia and Ambrosia hypersensitivity: co-sensitization or co-recognition? Clin Exp Allergy. 2006; 36:658–665.
Article28. Gadermaier G, Wopfner N, Wallner M, Egger M, Didierlaurent A, Regl G, et al. Array-based profiling of ragweed and mugwort pollen allergens. Allergy. 2008; 63:1543–1549.
Article29. Müller UR, Johansen N, Petersen AB, Fromberg-Nielsen J, Haeberli G. Hymenoptera venom allergy: analysis of double positivity to honey bee and Vespula venom by estimation of IgE antibodies to species-specific major allergens Api m1 and Ves v5. Allergy. 2009; 64:543–548.
Article30. Hemmer W, Focke M, Kolarich D, Wilson IB, Altmann F, Wöhrl S, et al. Antibody binding to venom carbohydrates is a frequent cause for double positivity to honeybee and yellow jacket venom in patients with stinging-insect allergy. J Allergy Clin Immunol. 2001; 108:1045–1052.
Article31. Müller U, Schmid-Grendelmeier P, Hausmann O, Helbling A. IgE to recombinant allergens Api m 1, Ves v 1, and Ves v 5 distinguish double sensitization from crossreaction in venom allergy. Allergy. 2012; 67:1069–1073.
Article32. Shreffler WG. Microarrayed recombinant allergens for diagnostic testing. J Allergy Clin Immunol. 2011; 127:843–849.
Article33. Arbes SJ Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005; 116:377–383.
Article34. Codreanu F, Collignon O, Roitel O, Thouvenot B, Sauvage C, Vilain AC, et al. A novel immunoassay using recombinant allergens simplifies peanut allergy diagnosis. Int Arch Allergy Immunol. 2011; 154:216–226.
Article35. Cromwell O, Häfner D, Nandy A. Recombinant allergens for specific immunotherapy. J Allergy Clin Immunol. 2011; 127:865–872.
Article36. Allergome [Internet]. Italy: Allergome;2013. Available from: http://www.allergome.org.37. Termo Fisher Scientific. ImmunoCAP Allergen list [Internet]. Sweden: Termo Fisher Scientific;2013. Available from: http://www.phadia.com/en/Products/Allergy-testing-products/ImmunoCAP-Allergen-Information/.38. Javaloyes G, Goikoetxea MJ, García Núñez I, Sanz ML, Blanca M, Scheurer S, et al. Performance of different in vitro techniques in the molecular diagnosis of peanut allergy. J Investig Allergol Clin Immunol. 2012; 22:508–513.39. Wöhrl S, Vigl K, Zehetmayer S, Hiller R, Jarisch R, Prinz M, et al. The performance of a component-based allergen-microarray in clinical practice. Allergy. 2006; 61:633–639.
Article40. Ebo DG, Bridts CH, Verweij MM, De Knop KJ, Hagendorens MM, De Clerck LS, et al. Sensitization profiles in birch pollen-allergic patients with and without oral allergy syndrome to apple: lessons from multiplexed component-resolved allergy diagnosis. Clin Exp Allergy. 2010; 40:339–347.
Article41. Villalta D, Asero R. Is the detection of IgE to multiple Bet v 1-homologous food allergens by means of allergen microarray clinically useful? J Allergy Clin Immunol. 2010; 125:1158–1161.
Article42. Bublin M, Dennstedt S, Buchegger M, Antonietta Ciardiello M, Bernardi ML, Tuppo L, et al. The performance of a component-based allergen microarray for the diagnosis of kiwifruit allergy. Clin Exp Allergy. 2011; 41:129–136.
Article43. Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010; 125:191–197.
Article44. Ebo DG, Hagendorens MM, De Knop KJ, Verweij MM, Bridts CH, De Clerck LS, et al. Component-resolved diagnosis from latex allergy by microarray. Clin Exp Allergy. 2010; 40:348–358.
Article45. Sastre J, Landivar ME, Ruiz-García M, Andregnette-Rosigno MV, Mahillo I. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy. 2012; 67:709–711.
Article46. Andersson K, Lidholm J. Characteristics and immunobiology of grass pollen allergens. Int Arch Allergy Immunol. 2003; 130:87–107.
Article47. Sekerkova A, Polackova M, Striz I. Detection of Phl p 1, Phl p 5, Phl p 7 and Phl p 12 specific IgE antibodies in the sera of children and adult patients allergic to Phleum pollen. Allergol Int. 2012; 61:339–346.
Article48. Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol Med. 2010; 16:321–328.
Article49. Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008; 122:951–960.
Article50. Larenas-Linnemann D. Oralair Birch, a recombinant major birch pollen allergen tablet for sublingual immunotherapy of allergic rhinitis caused by birch pollen. Curr Opin Investig Drugs. 2010; 11:586–596.51. Klimek L, Schendzielorz P, Pinol R, Pfaar O. Specific subcutaneous immunotherapy with recombinant grass pollen allergens: first randomized dose-ranging safety study. Clin Exp Allergy. 2012; 42:936–945.
Article52. Vrtala S, Hirtenlehner K, Vangelista L, Pastore A, Eichler HG, Sperr WR, et al. Conversion of the major birch pollen allergen, Bet v 1, into two nonanaphylactic T cell epitope-containing fragments: candidates for a novel form of specific immunotherapy. J Clin Invest. 1997; 99:1673–1681.
Article53. Vrtala S, Hirtenlehner K, Susani M, Akdis M, Kussebi F, Akdis CA, et al. Genetic engineering of a hypoallergenic trimer of the major birch pollen allergen Bet v 1. FASEB J. 2001; 15:2045–2047.
Article54. Purohit A, Niederberger V, Kronqvist M, Horak F, Grönneberg R, Suck R, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008; 38:1514–1525.
Article55. Kahlert H, Suck R, Weber B, Nandy A, Wald M, Keller W, et al. Characterization of a hypoallergenic recombinant Bet v 1 variant as a candidate for allergen-specific immunotherapy. Int Arch Allergy Immunol. 2008; 145:193–206.
Article56. Meyer W, Narkus A, Salapatek AM, Häfner D. Double-blind, placebo-controlled, dose-ranging study of new recombinant hypoallergenic Bet v 1 in an environmental exposure chamber. Allergy. 2013; 68:724–731.
Article57. Zuidmeer-Jongejan L, Fernandez-Rivas M, Poulsen LK, Neubauer A, Asturias J, Blom L, et al. FAST: towards safe and effective subcutaneous immunotherapy of persistent life-threatening food allergies. Clin Transl Allergy. 2012; 2:5.
Article58. Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, et al. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch Biochem Biophys. 1997; 342:244–253.
Article59. Burks AW, Shin D, Cockrell G, Stanley JS, Helm RM, Bannon GA. Mapping and mutational analysis of the IgE-binding epitopes on Ara h 1, a legume vicilin protein and a major allergen in peanut hypersensitivity. Eur J Biochem. 1997; 245:334–339.
Article60. Wood RA, Sicherer SH, Burks AW, Grishin A, Henning AK, Lindblad R, et al. A phase 1 study of heat/phenol-killed, E. coli-encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 (EMP-123) for the treatment of peanut allergy. Allergy. 2013; 68:803–808.
Article61. Moldaver D, Larché M. Immunotherapy with peptides. Allergy. 2011; 66:784–791.
Article62. Fellrath JM, Kettner A, Dufour N, Frigerio C, Schneeberger D, Leimgruber A, et al. Allergen-specific T-cell tolerance induction with allergen-derived long synthetic peptides: results of a phase I trial. J Allergy Clin Immunol. 2003; 111:854–861.
Article63. Pellaton C, Perrin Y, Boudousquié C, Barbier N, Wassenberg J, Corradin G, et al. Novel birch pollen specific immunotherapy formulation based on contiguous overlapping peptides. Clin Transl Allergy. 2013; 3:17.
Article64. Marth K, Breyer I, Focke-Tejkl M, Blatt K, Shamji MH, Layhadi J, et al. A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J Immunol. 2013; 190:3068–3078.
Article65. Twaroch TE, Focke M, Civaj V, Weber M, Balic N, Mari A, et al. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J Allergy Clin Immunol. 2011; 128:178–184.
Article66. Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010; 40:385–397.
Article67. Worm M, Lee HH, Kleine-Tebbe J, Hafner RP, Laidler P, Healey D, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011; 127:89–97.
Article68. Patel D, Couroux P, Hickey P, Salapatek AM, Laidler P, Larché M, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013; 131:103–109.69. Müller U, Akdis CA, Fricker M, Akdis M, Blesken T, Bettens F, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998; 101(6 Pt 1):747–754.
Article70. Hauser M, Asam C, Himly M, Palazzo P, Voltolini S, Montanari C, et al. Bet v 1-like pollen allergens of multiple Fagales species can sensitize atopic individuals. Clin Exp Allergy. 2011; 41:1804–1814.
Article71. Jahn-Schmid B, Harwanegg C, Hiller R, Bohle B, Ebner C, Scheiner O, et al. Allergen microarray: comparison of microarray using recombinant allergens with conventional diagnostic methods to detect allergen-specific serum immunoglobulin E. Clin Exp Allergy. 2003; 33:1443–1449.
Article72. Cabrera-Freitag P, Goikoetxea MJ, Beorlegui C, Gamboa P, Gastaminza G, Fernández-Benítez M, et al. Can component-based microarray replace fluorescent enzimoimmunoassay in the diagnosis of grass and cypress pollen allergy? Clin Exp Allergy. 2011; 41:1440–1446.
Article73. Bronnert M, Mancini J, Birnbaum J, Agabriel C, Liabeuf V, Porri F, et al. Component-resolved diagnosis with commercially available D. pteronyssinus Der p 1, Der p 2 and Der p 10: relevant markers for house dust mite allergy. Clin Exp Allergy. 2012; 42:1406–1415.
Article74. Ott H, Baron JM, Heise R, Ocklenburg C, Stanzel S, Merk HF, et al. Clinical usefulness of microarray-based IgE detection in children with suspected food allergy. Allergy. 2008; 63:1521–1528.
Article75. D'Urbano LE, Pellegrino K, Artesani MC, Donnanno S, Luciano R, Riccardi C, et al. Performance of a component-based allergen-microarray in the diagnosis of cow's milk and hen's egg allergy. Clin Exp Allergy. 2010; 40:1561–1570.76. Alessandri C, Zennaro D, Scala E, Ferrara R, Bernardi ML, Santoro M, et al. Ovomucoid (Gal d 1) specific IgE detected by microarray system predict tolerability to boiled hen's egg and an increased risk to progress to multiple environmental allergen sensitisation. Clin Exp Allergy. 2012; 42:441–450.
Article77. Hofmann SC, Fischer J, Eriksson C, Bengtsson Gref O, Biedermann T, Jakob T. IgE detection to α/β/γ-gliadin and its clinical relevance in wheat-dependent exercise-induced anaphylaxis. Allergy. 2012; 67:1457–1460.
Article78. Berneder M, Bublin M, Hoffmann-Sommergruber K, Hawranek T, Lang R. Allergen chip diagnosis for soy-allergic patients: Gly m 4 as a marker for severe food-allergic reactions to soy. Int Arch Allergy Immunol. 2013; 161:229–233.
Article79. Schuler S, Ferrari G, Schmid-Grendelmeier P, Harr T. Microarray-based component-resolved diagnosis of latex allergy: isolated IgE-mediated sensitization to latexprofilin Hev b8 may act as confounder. Clin Transl Allergy. 2013; 3:11.
Article80. Ott H, Schröder C, Raulf-Heimsoth M, Mahler V, Ocklenburg C, Merk HF, et al. Microarrays of recombinant Hevea brasiliensis proteins: a novel tool for the component-resolved diagnosis of natural rubber latex allergy. J Investig Allergol Clin Immunol. 2010; 20:129–138.81. Winther L, Poulsen LK, Robin B, Melac M, Malling HJ. Safety and tolerability of recombinant Bet v 1 (rBet v 1) tablets in sublingual immunotherapy (SLIT) [abstract]. J Allergy Clin Immunol. 2009; 123:Suppl. S215.82. Reisinger J, Horak F, Pauli G, van Hage M, Cromwell O, König F, et al. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J Allergy Clin Immunol. 2005; 116:347–354.
Article83. Niederberger V, Reisinger J, Valent P, Krauth MT, Pauli G, van Hage M, et al. Vaccination with genetically modified birch pollen allergens: immune and clinical effects on oral allergy syndrome. J Allergy Clin Immunol. 2007; 119:1013–1016.
Article84. Pree I, Shamji MH, Kimber I, Valenta R, Durham SR, Niederberger V. Inhibition of CD23-dependent facilitated allergen binding to B cells following vaccination with genetically modified hypoallergenic Bet v 1 molecules. Clin Exp Allergy. 2010; 40:1346–1352.
Article85. Pauli G, Purohit A, Oster JP, De Blay F, Vrtala S, Niederberger V, et al. Comparison of genetically engineered hypoallergenic rBet v 1 derivatives with rBet v 1 wild-type by skin prick and intradermal testing: results obtained in a French population. Clin Exp Allergy. 2000; 30:1076–1084.
Article86. Senti G, Crameri R, Kuster D, Johansen P, Martinez-Gomez JM, Graf N, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012; 129:1290–1296.
Article87. Zhang K, Zhu D, Kepley C, Terada T, Saxon A. Chimeric human fcgamma-allergen fusion proteins in the prevention of allergy. Immunol Allergy Clin North Am. 2007; 27:93–103.88. Maguire P, Nicodemus C, Robinson D, Aaronson D, Umetsu DT. The safety and efficacy of ALLERVAX CAT in cat allergic patients. Clin Immunol. 1999; 93:222–231.
Article89. Alexander C, Tarzi M, Larché M, Kay AB. The effect of Fel d 1-derived T-cell peptides on upper and lower airway outcome measurements in cat-allergic subjects. Allergy. 2005; 60:1269–1274.
Article90. Kündig TM, Senti G, Schnetzler G, Wolf C, Prinz Vavricka BM, Fulurija A, et al. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J Allergy Clin Immunol. 2006; 117:1470–1476.
Article