Korean J Radiol.
2015 Oct;16(5):1068-1078. 10.3348/kjr.2015.16.5.1068.
Does Establishing a Safety Margin Reduce Local Recurrence in Subsegmental Transarterial Chemoembolization for Small Nodular Hepatocellular Carcinomas?
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Seoul 03080, Korea. chungjw@snu.ac.kr
- 2Department of Radiology, Seoul National University College of Medicine, Seoul 03080, Korea.
- 3Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul 03080, Korea.
- 4Biomedical Research Institute, Seoul National University Hospital, Seoul 03080, Korea.
- 5Department of Radiology, Sheikh Khalifa Specialty Hospital, Ras Al Khaimah, United Arab Emirates.
- KMID: 2160774
- DOI: http://doi.org/10.3348/kjr.2015.16.5.1068
Abstract
OBJECTIVE
To test the hypothesis that a safety margin may affect local tumor recurrence (LTR) in subsegmental chemoembolization.
MATERIALS AND METHODS
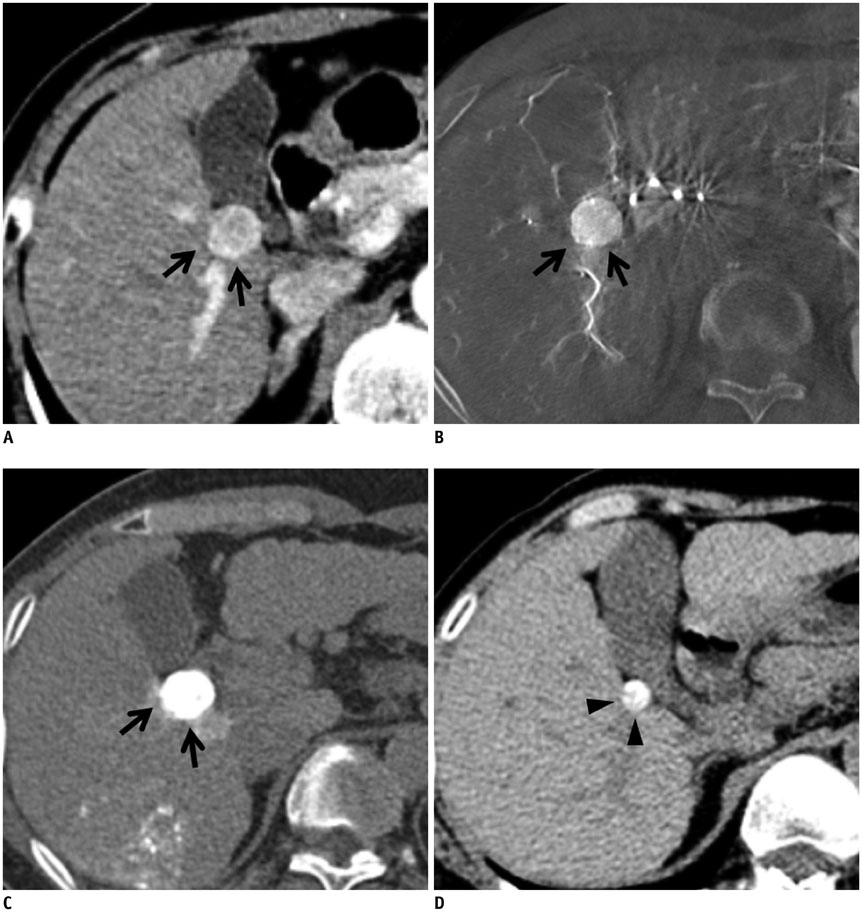
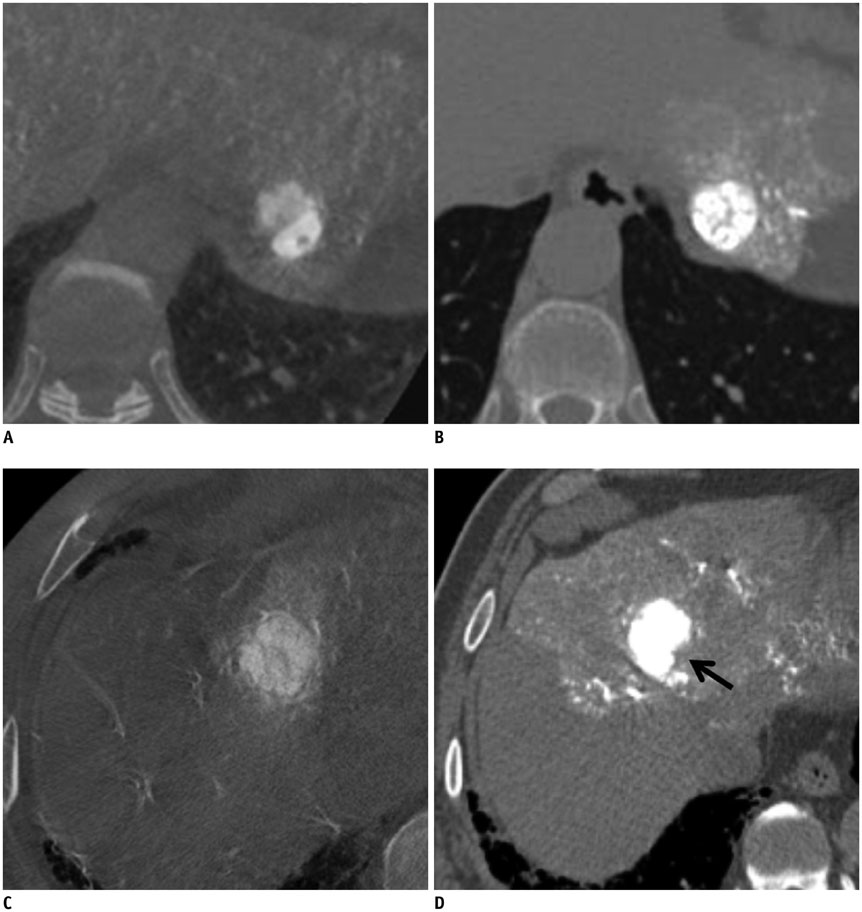
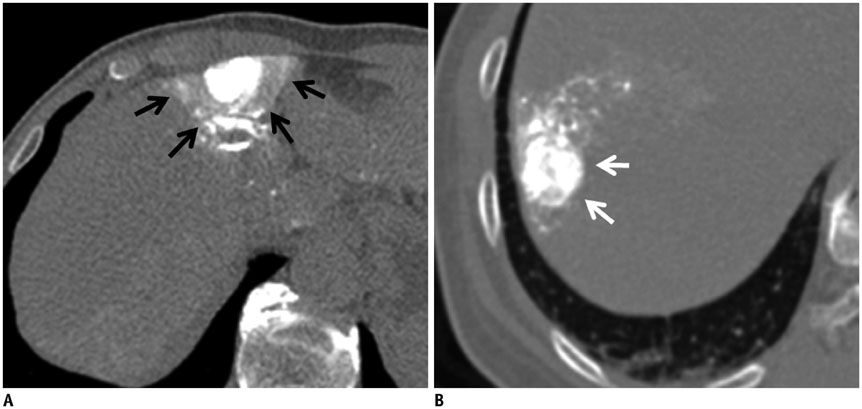
In 101 patients with 128 hepatocellular carcinoma (HCC) nodules (1-3 cm in size and < or = 3 in number), cone-beam CT-assisted subsegmental lipiodol chemoembolization was performed. Immediately thereafter, a non-contrast thin-section CT image was obtained to evaluate the presence or absence of intra-tumoral lipiodol uptake defect and safety margin. The effect of lipiodol uptake defect and safety margin on LTR was evaluated. Univariate and multivariate analyses were performed to indentify determinant factors of LTR.
RESULTS
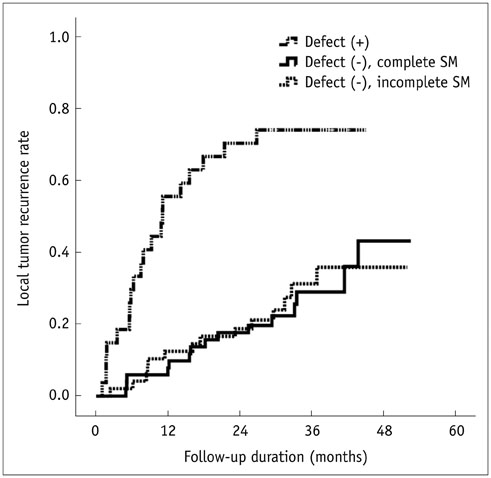
Of the 128 HCC nodules in 101 patients, 49 (38.3%) nodules in 40 patients showed LTR during follow-up period (median, 34.1 months). Cumulative 1- and 2-year LTR rates of nodules with lipiodol uptake defect (n = 27) and those without defect (n = 101) were 58.1% vs. 10.1% and 72.1% vs. 19.5%, respectively (p < 0.001). Among the 101 nodules without a defect, the 1- and 2-year cumulative LTR rates for nodules with complete safety margin (n = 52) and those with incomplete safety margin (n = 49) were 9.8% vs. 12.8% and 18.9% vs. 19.0% (p = 0.912). In multivariate analyses, ascites (p = 0.035), indistinct tumor margin on cone-beam CT (p = 0.039), heterogeneous lipiodol uptake (p = 0.023), and intra-tumoral lipiodol uptake defect (p < 0.001) were determinant factors of higher LTR.
CONCLUSION
In lipiodol chemoembolization, the safety margin in completely lipiodolized nodule without defect will not affect LTR in small nodular HCCs.
Keyword
MeSH Terms
-
Adult
Aged
Carcinoma, Hepatocellular/radiography/*therapy
Chemoembolization, Therapeutic
Cone-Beam Computed Tomography
Ethiodized Oil/*administration & dosage
Female
Follow-Up Studies
Humans
Liver Neoplasms/radiography/*therapy
Male
Middle Aged
Multivariate Analysis
Neoplasm Recurrence, Local/radiography
Ethiodized Oil
Figure
Cited by 2 articles
-
Comparison of the Efficacy and Prognostic Factors of Transarterial Chemoembolization Plus Microwave Ablation versus Transarterial Chemoembolization Alone in Patients with a Large Solitary or Multinodular Hepatocellular Carcinomas
Lin Zheng, Hai-Liang Li, Chen-Yang Guo, Su-Xia Luo
Korean J Radiol. 2018;19(2):237-246. doi: 10.3348/kjr.2018.19.2.237.Microvascular Flow Imaging of Residual or Recurrent Hepatocellular Carcinoma after Transarterial Chemoembolization: Comparison with Color/Power Doppler Imaging
Hyo-Jin Kang, Jeong Min Lee, Sun Kyung Jeon, Hwaseong Ryu, Jeongin Yoo, Jae Keun Lee, Joon Koo Han
Korean J Radiol. 2019;20(7):1114-1123. doi: 10.3348/kjr.2018.0932.
Reference
-
1. Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006; 243:229–235.2. Zytoon AA, Ishii H, Murakami K, El-Kholy MR, Furuse J, El-Dorry A, et al. Recurrence-free survival after radiofrequency ablation of hepatocellular carcinoma. A registry report of the impact of risk factors on outcome. Jpn J Clin Oncol. 2007; 37:658–672.3. Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013; 58:89–97.4. Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007; 245:36–43.5. Tang YH, Wen TF, Chen X. Resection margin in hepatectomy for hepatocellular carcinoma: a systematic review. Hepatogastroenterology. 2012; 59:1393–1397.6. Kim KW, Lee JM, Klotz E, Kim SJ, Kim SH, Kim JY, et al. Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and postprocedure CT images. AJR Am J Roentgenol. 2011; 196:W565–W572.7. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.8. Lee IJ, Chung JW, Yin YH, Kim HC, Kim YI, Jae HJ, et al. Cone-beam CT hepatic arteriography in chemoembolization for hepatocellular carcinoma: angiographic image quality and its determining factors. J Vasc Interv Radiol. 2014; 25:1369–1379. quiz 1379-1379.e19. Choi WS, Kim HC, Hur S, Choi JW, Lee JH, Yu SJ, et al. Role of C-arm CT in identifying caudate arteries supplying hepatocellular carcinoma. J Vasc Interv Radiol. 2014; 25:1380–1388.10. Kim HC. Role of C-arm cone-beam CT in chemoembolization for hepatocellular carcinoma. Korean J Radiol. 2015; 16:114–124.11. Song SY, Chung JW, Yin YH, Jae HJ, Kim HC, Jeon UB, et al. Celiac axis and common hepatic artery variations in 5002 patients: systematic analysis with spiral CT and DSA. Radiology. 2010; 255:278–288.12. Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007; 18:365–376.13. Ito K, Mitchell DG, Siegelman ES. Cirrhosis: MR imaging features. Magn Reson Imaging Clin N Am. 2002; 10:75–92. vi14. Gupta AA, Kim DC, Krinsky GA, Lee VS. CT and MRI of cirrhosis and its mimics. AJR Am J Roentgenol. 2004; 183:1595–1601.15. Lafortune M, Matricardi L, Denys A, Favret M, Déry R, Pomier-Layrargues G. Segment 4 (the quadrate lobe): a barometer of cirrhotic liver disease at US. Radiology. 1998; 206:157–160.16. de Franchis R. Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010; 53:762–768.17. de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001; 5:645–663.18. Annet L, Materne R, Danse E, Jamart J, Horsmans Y, Van Beers BE. Hepatic flow parameters measured with MR imaging and Doppler US: correlations with degree of cirrhosis and portal hypertension. Radiology. 2003; 229:409–414.19. Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005; 103:299–306.20. Nonami T, Harada A, Kurokawa T, Nakao A, Takagi H. Hepatic resection for hepatocellular carcinoma. Am J Surg. 1997; 173:288–291.21. Nagasue N, Uchida M, Makino Y, Takemoto Y, Yamanoi A, Hayashi T, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993; 105:488–494.22. Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000; 231:544–551.23. Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985; 161:346–350.24. Arii S, Tanaka S, Mitsunori Y, Nakamura N, Kudo A, Noguchi N, et al. Surgical strategies for hepatocellular carcinoma with special reference to anatomical hepatic resection and intraoperative contrast-enhanced ultrasonography. Oncology. 2010; 78:Suppl 1. 125–130.25. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009; 20:7 Suppl. S377–S390.26. Fujioka C, Horiguchi J, Ishifuro M, Kakizawa H, Kiguchi M, Matsuura N, et al. A feasibility study: evaluation of radiofrequency ablation therapy to hepatocellular carcinoma using image registration of preoperative and postoperative CT. Acad Radiol. 2006; 13:986–994.27. Miyayama S, Yamashiro M, Hashimoto M, Hashimoto N, Ikuno M, Okumura K, et al. Comparison of local control in transcatheter arterial chemoembolization of hepatocellular carcinoma ≤6 cm with or without intraprocedural monitoring of the embolized area using cone-beam computed tomography. Cardiovasc Intervent Radiol. 2014; 37:388–395.28. Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, et al. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991; 178:493–497.29. Takayasu K, Wakao F, Moriyama N, Muramatsu Y, Sakamoto M, Hirohashi S, et al. Response of early-stage hepatocellular carcinoma and borderline lesions to therapeutic arterial embolization. AJR Am J Roentgenol. 1993; 160:301–306.30. Miyayama S, Matsui O, Yamashiro M, Ryu Y, Takata H, Takeda T, et al. Iodized oil accumulation in the hypovascular tumor portion of early-stage hepatocellular carcinoma after ultraselective transcatheter arterial chemoembolization. Hepatol Int. 2007; 1:451–459.31. Wallace MJ, Murthy R, Kamat PP, Moore T, Rao SH, Ensor J, et al. Impact of C-arm CT on hepatic arterial interventions for hepatic malignancies. J Vasc Interv Radiol. 2007; 18:1500–1507.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Does Establishing a Safety Margin Reduce Local Recurrence in Subsegmental Transarterial Chemoembolization for Small Nodular Hepatocellular Carcinomas?
- Local Recurrence of Hepatocellular Carcinoma after Segmental Transarterial Chemoembolization: Risk Estimates Based on Multiple Prognostic Factors
- Safety margin of embolized area can reduce local recurrence of hepatocellular carcinoma after superselective transarterial chemoembolization
- Efficacy of Hepatic Arterial Infusion Chemotherapy and Radiofrequency Ablation against Hepatocellular Carcinoma Refractory to Transarterial Chemoembolization and Vascular Variation: A Case Study
- Risk Factors for Local Tumor Recurrence after Segmental Transarterial Chemoembolization for Hepatocellular Carcinoma: the Importance of Tumor Located in the Segmental Border Zone