Hyperfunction Thyroid Nodules: Their Risk for Becoming or Being Associated with Thyroid Cancers
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Seoul 110-744, Korea. jihnkim@gmail.com
- 2Human Medical Imaging & Intervention Center, Seoul 137-902, Korea.
- 3Department of Nuclear Medicine, Seoul National University Hospital, Seoul 110-744, Korea.
- 4Department of Pathology, Seoul National University Hospital, Seoul 110-744, Korea.
- 5Department of Radiology, Soonchunhyang University Bucheon Hospital, Bucheon 420-767, Korea.
- KMID: 1715770
- DOI: http://doi.org/10.3348/kjr.2013.14.4.643
Abstract
OBJECTIVE
To retrospectively evaluate the risk of thyroid cancer in patients with hyperfunctioning thyroid nodules through ultrasonographic-pathologic analysis.
MATERIALS AND METHODS
Institutional review board approval was obtained and informed consent was waived. From 2003 to 2007, 107 patients consecutively presented with hot spots on thyroid scans and low serum thyroid-stimulating hormone levels. Among them, 32 patients who had undergone thyroid ultrasonography were analyzed in this study. Thyroid nodules depicted on ultrasonography were classified based on size and categorized as benign, indeterminate, or suspicious malignant nodules according to ultrasonographic findings. The thyroid nodules were determined as either hyperfunctioning or coexisting nodules and were then correlated with pathologic results.
RESULTS
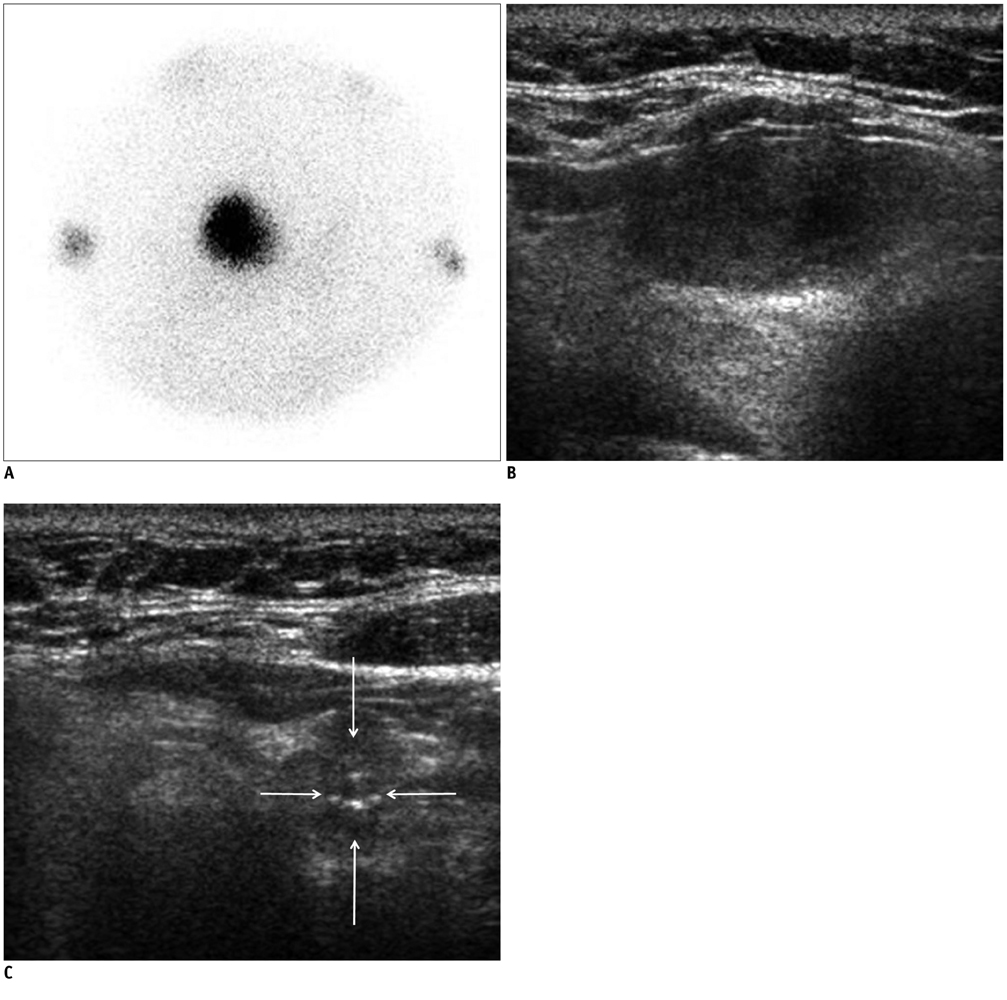
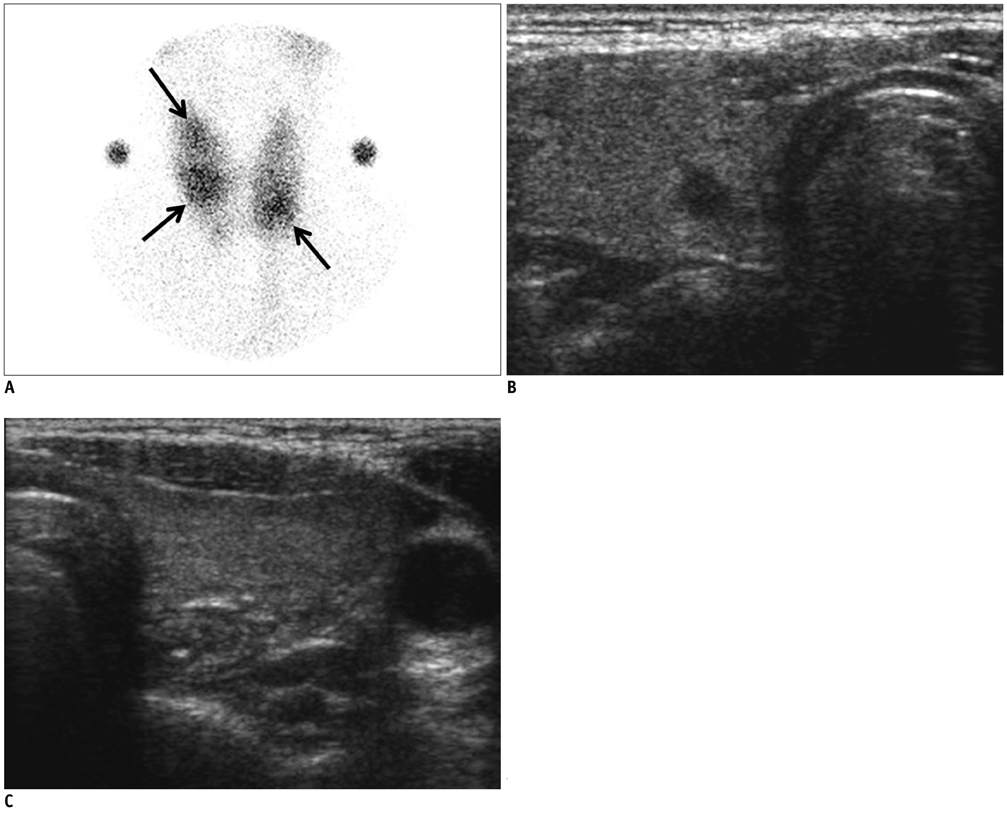
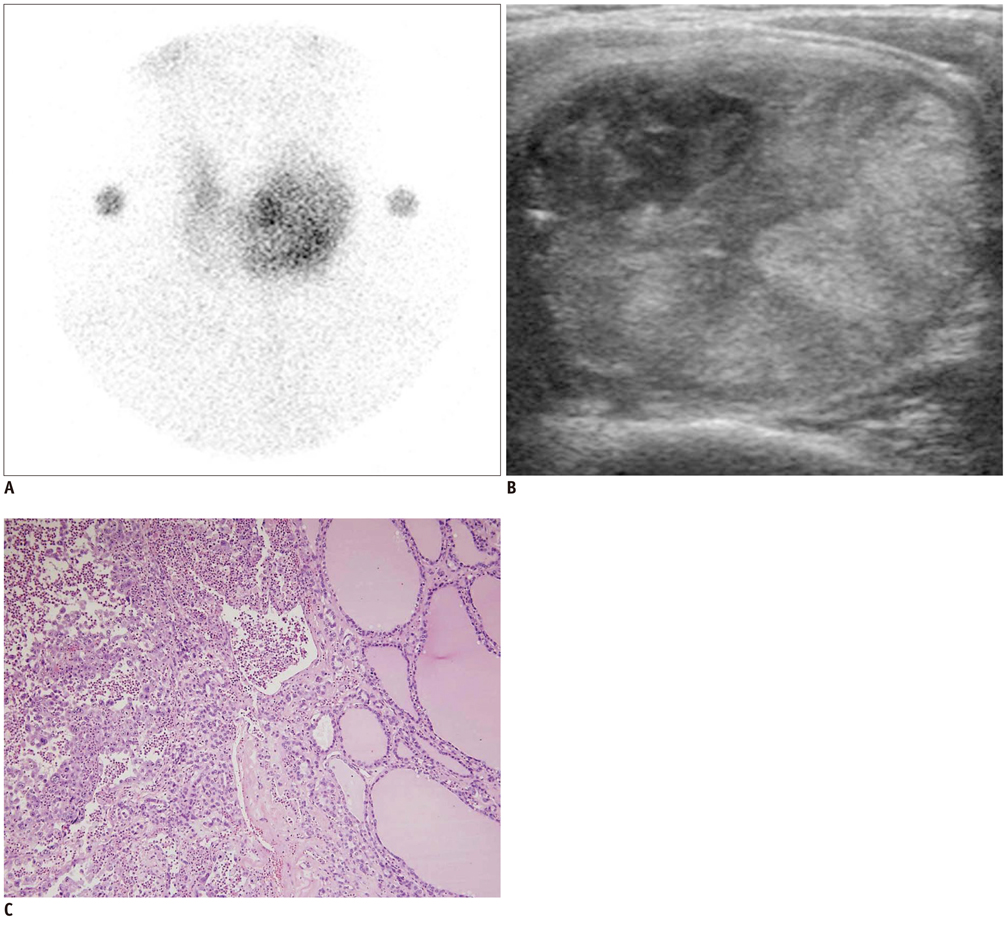
In 32 patients, 42 hyperfunctioning nodules (mean number per patient, 1.31; range, 1-6) were observed on thyroid scans and 68 coexisting nodules (mean, 2.13; range, 0-7) were observed on ultrasonography. Twenty-five patients (78.1%) had at least one hyperfunctioning (n = 17, 53.1%) or coexisting (n = 16, 50.0%) nodule that showed a suspicious malignant feature larger than 5 mm (n = 8, 25.0%), or an indeterminate feature 1 cm or greater (n = 20, 62.5%) in diameter, which could have been indicated by using fine needle aspiration (FNA). Seven patients were proven to have 11 thyroid cancers in 3 hyperfunctioning and 8 coexisting nodules. All of these had at least one thyroid cancer, which could have been indicated by using FNA. The estimated minimal risk of thyroid cancer was 6.5% (7/107).
CONCLUSION
Patients with hyperfunctioning nodules may not be safe from thyroid cancer because hyperfunctioning nodules can coexist with thyroid cancer nodules. To screen out these cancers, ultrasonography should be performed.
MeSH Terms
Figure
Cited by 3 articles
-
Ultrasonographic Characteristics of the Hyperfunctioning Thyroid Nodule and Predictive Factors for Thyroid Stimulating Hormone Suppression
Won Sang Yoo, Hoon Sung Choi
Int J Thyroidol. 2019;12(1):35-43. doi: 10.11106/ijt.2019.12.1.35.2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology
Ji-hoon Kim, Jung Hwan Baek, Hyun Kyung Lim, Hye Shin Ahn, Seon Mi Baek, Yoon Jung Choi, Young Jun Choi, Sae Rom Chung, Eun Ju Ha, Soo Yeon Hahn, So Lyung Jung, Dae Sik Kim, Soo Jin Kim, Yeo Koon Kim, Chang Yoon Lee, Jeong Hyun Lee, Kwang Hwi Lee, Young Hen Lee, Jeong Seon Park, Hyesun Park, Jung Hee Shin, Chong Hyun Suh, Jin Yong Sung, Jung Suk Sim, Inyoung Youn, Miyoung Choi, Dong Gyu Na,
Korean J Radiol. 2018;19(4):632-655. doi: 10.3348/kjr.2018.19.4.632.Papillary Thyroid Carcinoma Presented as a Hot Nodule with Hyperthyroidism
Sung Hye Kong, Seo Young Lee, Ye Seul Yang, Jae Hoon Moon
Int J Thyroidol. 2016;9(1):47-50. doi: 10.11106/ijt.2016.9.1.47.
Reference
-
1. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.2. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010; 16:Suppl 1. 1–43.3. Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010; 8:1228–1274.4. Smith JR, Oates E. Radionuclide imaging of the thyroid gland: patterns, pearls, and pitfalls. Clin Nucl Med. 2004; 29:181–193.5. Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med. 2004; 351:1764–1771.6. Mandel SJ. A 64-year-old woman with a thyroid nodule. JAMA. 2004; 292:2632–2642.7. Harach HR, Sánchez SS, Williams ED. Pathology of the autonomously functioning (hot) thyroid nodule. Ann Diagn Pathol. 2002; 6:10–19.8. Als C, Gedeon P, Rösler H, Minder C, Netzer P, Laissue JA. Survival analysis of 19 patients with toxic thyroid carcinoma. J Clin Endocrinol Metab. 2002; 87:4122–4127.9. Majima T, Doi K, Komatsu Y, Itoh H, Fukao A, Shigemoto M, et al. Papillary thyroid carcinoma without metastases manifesting as an autonomously functioning thyroid nodule. Endocr J. 2005; 52:309–316.10. Tfayli HM, Teot LA, Indyk JA, Witchel SF. Papillary thyroid carcinoma in an autonomous hyperfunctioning thyroid nodule: case report and review of the literature. Thyroid. 2010; 20:1029–1032.11. Iwata M, Kasagi K, Hatabu H, Misaki T, Iida Y, Fujita T, et al. Causes of appearance of scintigraphic hot areas on thyroid scintigraphy analyzed with clinical features and comparative ultrasonographic findings. Ann Nucl Med. 2002; 16:279–287.12. David E, Rosen IB, Bain J, James J, Kirsh JC. Management of the hot thyroid nodule. Am J Surg. 1995; 170:481–483.13. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009; 19:1159–1165.14. Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011; 12:1–14.15. Yi KH, Park YJ, Koong SS, Kim JH, Na DG, Ryu JS, et al. Revised Korean thyroid association management guidelines for patients with thyroid nodules and thyroid cancer. Korean J Otorhinolaryngol-Head Neck Surg. 2011; 54:8–36.16. Reiners C, Schumm-Draeger PM, Geling M, Mastbaum C, Schönberger J, Laue-Savic A, et al. [Thyroid gland ultrasound screening (Papillon Initiative). Report of 15 incidentally detected thyroid cancers]. Internist (Berl). 2003; 44:412–419.17. Brander AE, Viikinkoski VP, Nickels JI, Kivisaari LM. Importance of thyroid abnormalities detected at US screening: a 5-year follow-up. Radiology. 2000; 215:801–806.18. Mizukami Y, Michigishi T, Nonomura A, Yokoyama K, Noguchi M, Hashimoto T, et al. Autonomously functioning (hot) nodule of the thyroid gland. A clinical and histopathologic study of 17 cases. Am J Clin Pathol. 1994; 101:29–35.19. Tan GH, Gharib H, Reading CC. Solitary thyroid nodule. Comparison between palpation and ultrasonography. Arch Intern Med. 1995; 155:2418–2423.20. Marqusee E, Benson CB, Frates MC, Doubilet PM, Larsen PR, Cibas ES, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000; 133:696–700.21. Kim DS, Kim JH, Na DG, Park SH, Kim E, Chang KH, et al. Sonographic features of follicular variant papillary thyroid carcinomas in comparison with conventional papillary thyroid carcinomas. J Ultrasound Med. 2009; 28:1685–1692.22. Sillery JC, Reading CC, Charboneau JW, Henrichsen TL, Hay ID, Mandrekar JN. Thyroid follicular carcinoma: sonographic features of 50 cases. AJR Am J Roentgenol. 2010; 194:44–54.23. Jeh SK, Jung SL, Kim BS, Lee YS. Evaluating the degree of conformity of papillary carcinoma and follicular carcinoma to the reported ultrasonographic findings of malignant thyroid tumor. Korean J Radiol. 2007; 8:192–197.24. Kusić Z, Becker DV, Saenger EL, Paras P, Gartside P, Wessler T, et al. Comparison of technetium-99m and iodine-123 imaging of thyroid nodules: correlation with pathologic findings. J Nucl Med. 1990; 31:393–399.25. Reschini E, Catania A, Ferrari C, Bergonzi M, Paracchi A, Raineri P. Comparison of pertechnetate and radioiodine thyroid scintiscans in thyroid disease. J Nucl Biol Med. 1993; 37:12–17.26. Belfiore A, Sava L, Runello F, Tomaselli L, Vigneri R. Solitary autonomously functioning thyroid nodules and iodine deficiency. J Clin Endocrinol Metab. 1983; 56:283–287.