Korean J Physiol Pharmacol.
2012 Dec;16(6):431-436. 10.4196/kjpp.2012.16.6.431.
Synergistic Induction of iNOS by IFN-gamma and Glycoprotein Isolated from Dioscorea batatas
- Affiliations
-
- 1Department of Pharmacology, School of Medicine, Chosun University, Gwangju 501-759, Korea. yjjeon@chosun.ac.kr
- 2Department of Anatomy, School of Medicine, Chosun University, Gwangju 501-759, Korea.
- 3Department of Thoracic and Cardiovascular Surgery, School of Medicine, Chosun University, Gwangju 501-759, Korea.
- 4Department of Anatomy, School of Medicine, Jeju National University, Jeju 690-756, Korea.
- 5Department of Biotechnology and Biomedicine, Chungbuk Provincial College, Chungbuk 373-806, Korea.
- KMID: 2285452
- DOI: http://doi.org/10.4196/kjpp.2012.16.6.431
Abstract
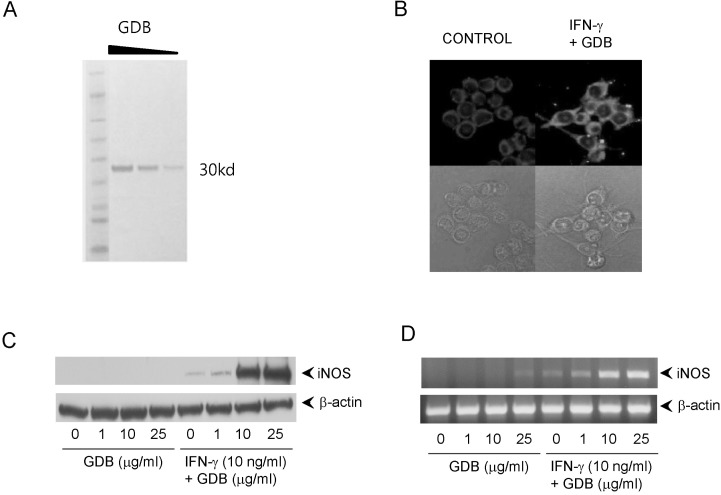
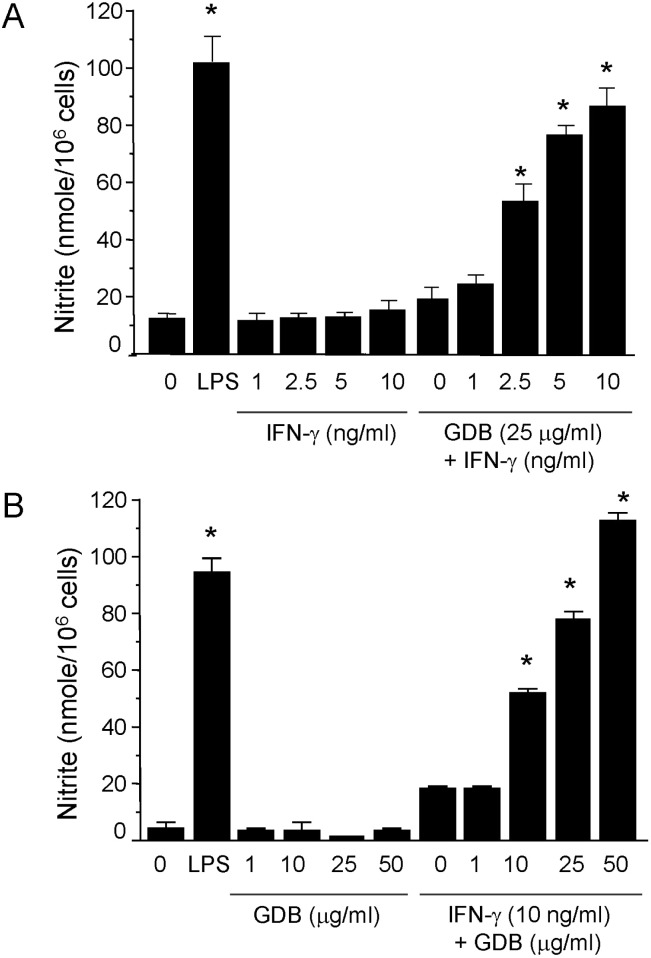
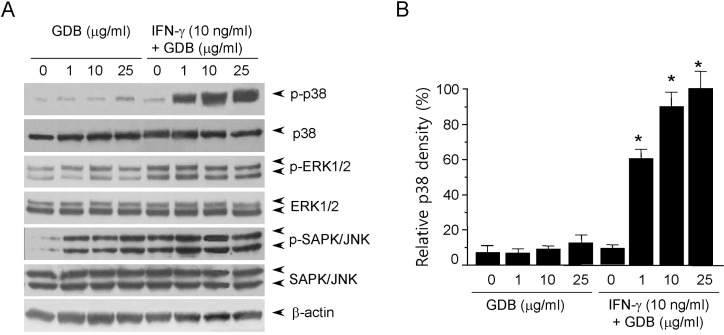
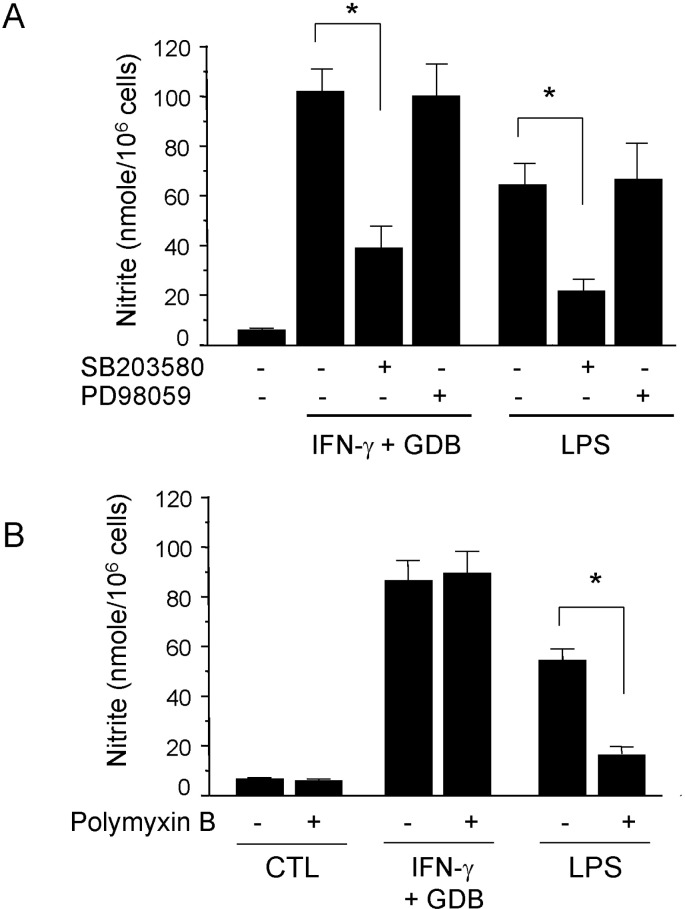
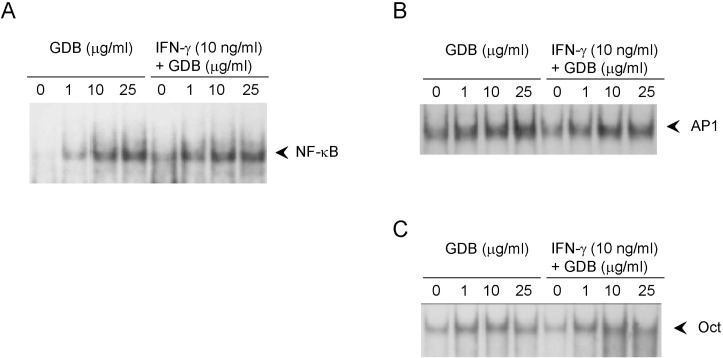
- Dioscorea species continue to be used in traditional Chinese medicine, and represent a major source of steroid precursors for conventional medicine. In the previous study, We isolated glycoprotein (GDB) from Dioscorea batatas, characterized, and demonstrated immunostimulating activity in C57BL/6 mice. The aim of this study was to investigate the mechanism whereby GDB activates macrophages. Macrophages activation by GDB was investigated by analyzing the effects of GDB on nitric oxide (NO) production, iNOS expression, mitogen activated protein kinase (MAPK) phosphorylation, and transcription factor activation. In the presence of IFN-gamma, GDB strongly stimulated macrophages to express iNOS and produce NO. Furthermore, the activation of p38 was synergistically induced by GDB plus IFN-gamma , but SB203580 (a p38 inhibitor) inhibited GDB plus IFN-gamma-induced p38 activation. This study indicates that GDB is an important activator of macrophages. Furthermore, due to the critical role that macrophage activation plays in innate immune response, the activation effects of GDB on macrophages suggest that GDB may be a useful immunopotentiating agent.
Keyword
MeSH Terms
Figure
Reference
-
1. Chen H, Wang C, Chang CT, Wang T. Effects of Taiwanese yam (Dioscorea japonica Thunb var. pseudojaponica Yamamoto) on upper gut function and lipid metabolism in Balb/c mice. Nutrition. 2003; 19:646–651. PMID: 12831952.
Article2. Jin UH, Kim DI, Lee TK, Lee DN, Kim JK, Lee IS, Kim CH. Herbal formulation, Yukmi-jihang-tang-Jahage, regulates bone resorption by inhibition of phosphorylation mediated by tyrosine kinase Src and cyclooxygenase expression. J Ethnopharmacol. 2006; 106:333–343. PMID: 16513308.
Article3. Lin PL, Lin KW, Weng CF, Lin KC. Yam storage protein dioscorins from Dioscorea alata and Dioscorea japonica exhibit distinct immunomodulatory activities in mice. J Agric Food Chem. 2009; 57:4606–4613. PMID: 19378946.
Article4. Fu SL, Hsu YH, Lee PY, Hou WC, Hung LC, Lin CH, Chen CM, Huang YJ. Dioscorin isolated from Dioscorea alata activates TLR4-signaling pathways and induces cytokine expression in macrophages. Biochem Biophys Res Commun. 2006; 339:137–144. PMID: 16297883.
Article5. Choi EM, Koo SJ, Hwang JK. Immune cell stimulating activity of mucopolysaccharide isolated from yam (Dioscorea batatas). J Ethnopharmacol. 2004; 91:1–6. PMID: 15036459.
Article7. Huong PT, Lee CH, Li MH, Lee MY, Kim JK, Lee SM, Seon JH, Lee DC, Jeon YJ. Characterization and immunopotentiating effects of the glycoprotein isolated from dioscorea batatas. Korean J Physiol Pharmacol. 2011; 15:101–106. PMID: 21660150.
Article8. Higuchi M, Higashi N, Taki H, Osawa T. Cytolytic mechanisms of activated macrophages. Tumor necrosis factor and L-arginine-dependent mechanisms act synergistically as the major cytolytic mechanisms of activated macrophages. J Immunol. 1990; 144:1425–1431. PMID: 2303713.9. Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989; 169:1543–1555. PMID: 2497225.
Article10. Billack B. Macrophage activation: role of toll-like receptors, nitric oxide, and nuclear factor kappa B. Am J Pharm Educ. 2006; 70:102. PMID: 17149431.
Article11. Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, Murphy WJ. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci USA. 1993; 90:9730–9734. PMID: 7692452.
Article13. Jeon YJ, Han SB, Ahn KS, Kim HM. Differential activation of murine macrophages by angelan and LPS. Immunopharmacology. 2000; 49:275–284. PMID: 10996025.
Article14. Ban JY, Kim BS, Kim SC, Kim DH, Chung JH. Microarray analysis of gene expression profiles in response to treatment with melatonin in lipopolysaccharide activated RAW 264.7 cells. Korean J Physiol Pharmacol. 2011; 15:23–29. PMID: 21461237.
Article15. Li MH, Kothandan G, Cho SJ, Huong PT, Nan YH, Lee KY, Shin SY, Yea SS, Jeon YJ. Magnolol Inhibits LPS-induced NF-κB/Rel Activation by Blocking p38 Kinase in Murine Macrophages. Korean J Physiol Pharmacol. 2010; 14:353–358. PMID: 21311674.
Article16. Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996; 8:402–411. PMID: 8793994.
Article17. Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995; 270:7420–7426. PMID: 7535770.
Article18. Weinstein SL, Sanghera JS, Lemke K, DeFranco AL, Pelech SL. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992; 267:14955–14962. PMID: 1321821.
Article19. Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med (Berl). 1996; 74:589–607. PMID: 8912180.
Article20. Gaidamashvili M, Ohizumi Y, Iijima S, Takayama T, Ogawa T, Muramoto K. Characterization of the yam tuber storage proteins from Dioscorea batatas exhibiting unique lectin activities. J Biol Chem. 2004; 279:26028–26035. PMID: 15047697.
Article21. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–138. PMID: 7181105.
Article22. Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993; 177:1779–1784. PMID: 7684434.
Article23. Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Statistics Assoc. 1995; 50:1096–1121.
Article24. Jeon YJ, Kim YK, Lee M, Park SM, Han SB, Kim HM. Radicicol suppresses expression of inducible nitric-oxide synthase by blocking p38 kinase and nuclear factor-kappaB/Rel in lipopolysaccharide-stimulated macrophages. J Pharmacol Exp Ther. 2000; 294:548–554. PMID: 10900231.25. Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997; 9:180–186. PMID: 9069255.
Article26. Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)--from inflammation to development. Curr Opin Cell Biol. 1998; 10:205–219. PMID: 9561845.27. Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984; 2:283–318. PMID: 6100475.
Article28. Hibbs JB Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987; 235:473–476. PMID: 2432665.
Article29. Thornton BP, Větvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J Immunol. 1996; 156:1235–1246. PMID: 8558003.30. Goyert SM, Ferrero E, Rettig WJ, Yenamandra AK, Obata F, Le Beau MM. The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science. 1988; 239:497–500. PMID: 2448876.
Article31. Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992; 69:11–25. PMID: 1555235.
Article32. Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999; 11:13–18. PMID: 10047546.
Article33. Oh PS, Lim KT. HeLa cells treated with phytoglycoprotein (150 kDa) were killed by activation of caspase 3 via inhibitory activities of NF-kappaB and AP-1. J Biomed Sci. 2007; 14:223–232. PMID: 17192825.34. Lee SJ, Lim KT. Phytoglycoprotein inhibits interleukin-1beta and interleukin-6 via p38 mitogen-activated protein kinase in lipopolysaccharide-stimulated RAW 264.7 cells. Naunyn Schmiedebergs Arch Pharmacol. 2008; 377:45–54. PMID: 18204996.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synergistic Induction of iNOS by IFN-gamma and Glycoprotein Isolated from Dioscorea batatas
- Characterization and Immunopotentiating Effects of the Glycoprotein Isolated from Dioscorea Batatas
- Identification of Dioscorea Batatas (Sanyak) Allergen as an Inhalant and Oral Allergen
- Diclofenac inhibits IFN-gamma plus lipopolysaccharide-induced iNOS gene expression via suppression of NF-kappaB activation in RAW 264.7 macrophages
- Expression of Inducible Nitric Oxide Synthase and Nitric Oxide Mediated Apoptosis in Neuronal PC12 Cells after Lipopolysaccharide/Tumor Necrosis Factor-/Interferon- Treatment