Changes in Serotype Distribution and Antibiotic Resistance of Nasopharyngeal Isolates of Streptococcus pneumoniae from Children in Korea, after Optional Use of the 7-Valent Conjugate Vaccine
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Children's Hospital, Seoul, Korea. eunchoi@snu.ac.kr
- 2Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Pediatrics, The Catholic University of Korea, Seoul St. Mary's Hospital, Seoul, Korea.
- KMID: 2157913
- DOI: http://doi.org/10.3346/jkms.2012.27.7.716
Abstract
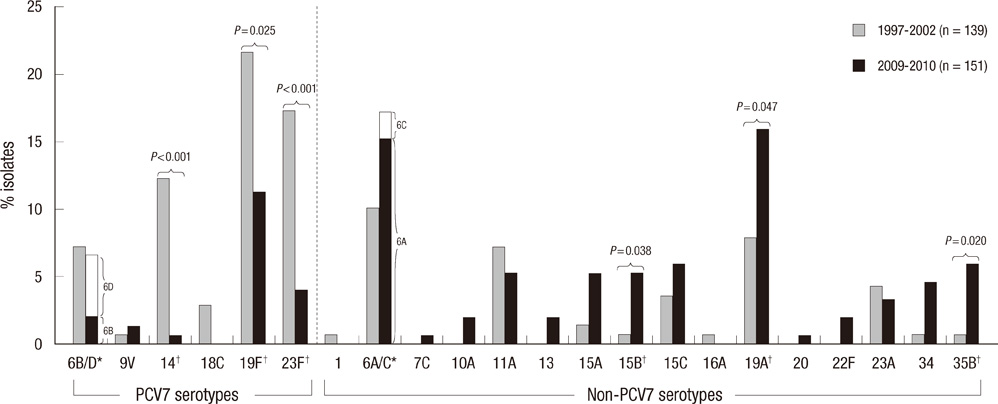
- We investigated serotype distribution and antimicrobial resistance of pneumococcal carriage isolates from children after optional immunization with the 7-valent pneumococcal conjugate vaccine (PCV7) in Korea. From June 2009 to June 2010, 205 (16.5%) pneumococcal isolates were obtained from 1,243 nasopharyngeal aspirates of infants and children at Seoul National University Children's Hospital, Korea. Serotype was determined by Quellung reaction and antibiotic susceptibility was tested by E-test. The results were compared to previous studies done in the pre-PCV7 period. In this study, the most common serotypes were 6A (15.3%), 19A (14.7%), 19F (10.2%), 35B (7.3%), and 6D (5.6%). The proportion of PCV7 serotypes decreased from 61.9% to 23.8% (P < 0.001). The overall penicillin nonsusceptibility rate increased from 83.5% to 95.4% (P = 0.001). This study demonstrates the impact of optional PCV7 vaccination in Korea; the proportion of all PCV7 serotypes except 19F decreased while antimicrobial resistant serotypes 6A and 19A further increased.
MeSH Terms
-
Anti-Bacterial Agents/pharmacology
Child, Preschool
Drug Resistance, Bacterial/drug effects
Humans
Infant
Microbial Sensitivity Tests
Nasopharynx/*microbiology
Pneumococcal Infections/immunology/prevention & control
Republic of Korea
Serotyping
Streptococcus pneumoniae/classification/*isolation & purification
Vaccination
Vaccines, Conjugate/*immunology
Anti-Bacterial Agents
Vaccines, Conjugate
Figure
Cited by 6 articles
-
The Changing Epidemiology of Childhood Pneumococcal Disease in Korea
Young June Choe, Eun Hwa Choi, Hoan Jong Lee
Infect Chemother. 2013;45(2):145-158. doi: 10.3947/ic.2013.45.2.145.Indirect Effects of Pneumococcal Conjugate Vaccines in National Immunization Programs for Children on Adult Pneumococcal Disease
Young Keun Kim, David LaFon, Moon H. Nahm
Infect Chemother. 2016;48(4):257-266. doi: 10.3947/ic.2016.48.4.257.Pneumococcal vaccine
Joon Young Song, Hee Jin Cheong
J Korean Med Assoc. 2014;57(9):780-788. doi: 10.5124/jkma.2014.57.9.780.Antibiotics Susceptability of
Streptococcus pneumoniae Isolated from Single Tertiary Childrens' Hospital Since 2014 and Choice of Appropriate Empirical Antibiotics
Jiwon Jung, Ree Nar Yoo, Hungseop Sung, Mina Kim, Jina Lee
Pediatr Infect Vaccine. 2019;26(1):1-10. doi: 10.14776/piv.2019.26.e1.The prevention of pneumococcal infections
Mee Soo Chang, Jun Hee Woo
Clin Exp Vaccine Res. 2016;5(1):3-5. doi: 10.7774/cevr.2016.5.1.3.Adult Immunization in Patients with Diabetes Mellitus: Current Immunization Status and Recommended Schedule in Korea
Eun-Jeong Joo, Joon-Sup Yeom
J Korean Diabetes. 2013;14(3):103-110. doi: 10.4093/jkd.2013.14.3.103.
Reference
-
1. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004. 4:144–154.2. Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, Jackson D, Thomas A, Beall B, Lynfield R, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007. 196:1346–1354.3. Centers for Disease Control and Prevention (CDC). Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction: eight states, 1998-2005. MMWR Morb Mortal Wkly Rep. 2008. 57:144–148.4. Moore MR, Hyde TB, Hennessy TW, Parks DJ, Reasonover AL, Harker-Jones M, Gove J, Bruden DL, Rudolph K, Parkinson A, et al. Impact of a conjugate vaccine on community-wide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. J Infect Dis. 2004. 190:2031–2038.5. Park SY, Moore MR, Bruden DL, Hyde TB, Reasonover AL, Harker-Jones M, Rudolph KM, Hurlburt DA, Parks DJ, Parkinson AJ, et al. Impact of conjugate vaccine on transmission of antimicrobial-resistant Streptococcus pneumoniae among Alaskan children. Pediatr Infect Dis J. 2008. 27:335–340.6. Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein J. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009. 124:e1–e11.7. Grivea IN, Panagiotou M, Tsantouli AG, Syrogiannopoulos GA. Impact of heptavalent pneumococcal conjugate vaccine on nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae among day-care center attendees in central Greece. Pediatr Infect Dis J. 2008. 27:519–525.8. Lee JA, Kim NH, Kim DH, Park KW, Kim YK, Kim KH, Park JY, Choi EH, Lee HJ. Serotypes and penicillin susceptibility of Streptococcus pneumoniae isolated from clinical specimens and healthy carriers of Korean children. J Korean Pediatr Soc. 2003. 46:846–853.9. Kim SM, Hur JK, Lee KY, Shin YK, Park SE, Ma SH, Min AY, Kang JH. Epidemiological study of pneumococcal nasal carriage and serotypes among Korean children. Korean J Pediatr. 2004. 47:611–616.10. Choi EH, Lee HJ, Cho EY, Oh CE, Eun BW, Lee J, Kim MJ. Prevalence and genetic structures of Streptococcus pneumoniae serotype 6D, South Korea. Emerg Infect Dis. 2010. 16:1751–1753.11. Clinical and Laboratory Standards Institute. CLSI document M100-S18. Performance standards for antimicrobial susceptibility testing: 18th information supplement. 2008. Wayne PA: National Committee for Clinical and Laboratory Standards.12. Dagan R, Klugman KP. Impact of conjugate pneumococcal vaccines on antibiotic resistance. Lancet Infect Dis. 2008. 8:785–795.13. Dunais B, Bruno-Bazureault P, Carsenti-Dellamonica H, Touboul P, Pradier C. A decade-long surveillance of nasopharyngeal colonisation with Streptococcus pneumoniae among children attending day-care centres in south-eastern France: 1999-2008. Eur J Clin Microbiol Infect Dis. 2011. 30:837–843.14. Grivea IN, Tsantouli AG, Michoula AN, Syrogiannopoulos GA. Dynamics of Streptococcus pneumoniae nasopharyngeal carriage with high heptavalent pneumococcal conjugate vaccine coverage in Central Greece. Vaccine. 2011. 29:8882–8887.15. Rodrigues F, Nunes S, Sá-Leão R, Gonçalves G, Lemos L, de Lencastre H. Streptococcus pneumoniae nasopharyngeal carriage in children attending day-care centers in the central region of Portugal, in the era of 7-valent pneumococcal conjugate vaccine. Microb Drug Resist. 2009. 15:269–277.16. Vestrheim DF, Høiby EA, Aaberge IS, Caugant DA. Impact of a pneumococcal conjugate vaccination program on carriage among children in Norway. Clin Vaccine Immunol. 2010. 17:325–334.17. Bryant KA, Block SL, Baker SA, Gruber WC, Scott DA. PCV13 Infant Study Group. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics. 2010. 125:866–875.18. Kieninger DM, Kueper K, Steul K, Juergens C, Ahlers N, Baker S, Jansen KU, Devlin C, Gruber WC, Emini EA, et al. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine. 2010. 28:4192–4203.19. Schuerman L, Wysocki J, Tejedor JC, Knuf M, Kim KH, Poolman J. Prediction of pneumococcal conjugate vaccine effectiveness against invasive pneumococcal disease using opsonophagocytic activity and antibody concentrations determined by enzyme-linked immunosorbent assay with 22F adsorption. Clin Vaccine Immunol. 2011. 18:2161–2167.20. Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010. 201:32–41.21. Choi EH, Kim SH, Eun BW, Kim SJ, Kim NH, Lee J, Lee HJ. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis. 2008. 14:275–281.22. Ho PL, Chiu SS, Chan MY, Ang I, Chow KH, Lau YL. Changes in nasopharyngeal carriage and serotype distribution of antibiotic-resistant Streptococcus pneumoniae before and after the introduction of 7-valent pneumococcal conjugate vaccine in Hong Kong. Diagn Microbiol Infect Dis. 2011. 71:327–334.23. Hanage WP, Bishop CJ, Huang SS, Stevenson AE, Pelton SI, Lipsitch M, Finkelstein JA. Carried pneumococci in Massachusetts children: the contribution of clonal expansion and serotype switching. Pediatr Infect Dis J. 2011. 30:302–308.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in Serotype Distribution and Antibiotic Resistance of Nasopharyngeal Isolates of Streptococcus pneumoniae from Children in Korea, after Optional Use of the 7-Valent Conjugate Vaccine
- Recommendation for use of the newly introduced pneumococcal protein conjugate vaccines in Korea
- Efficacy and effectiveness of pneumococcal conjugate vaccine in children
- Direct and Indirect Effects of Pneumococcal Protein Conjugate Vaccine
- Serotype and Antimicrobial Susceptibility of Streptococcus pneumoniae