Changing Molecular Epidemiology of Group B Streptococcus in Korea
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Eulji Hospital, Seoul, Korea.
- 2Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, MI. USA.
- 3Department of Obstetrics and Gynecology, Eulji University Hospital, Daejeon, Korea.
- 4Department of Laboratory Medicine, Eulji Hospital, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, Cheil General Hospital & Women's Healthcare Center, Kwandong University College of Medicine, Seoul, Korea.
- 6Seoul Clinical Laboratories & Seoul Medical Science Institute, Seoul, Korea.
- 7Department of Preventive Medicine, Eulji University School of Medicine, Daejeon, Korea. kimoran@eulji.ac.kr
- KMID: 2150857
- DOI: http://doi.org/10.3346/jkms.2010.25.6.817
Abstract
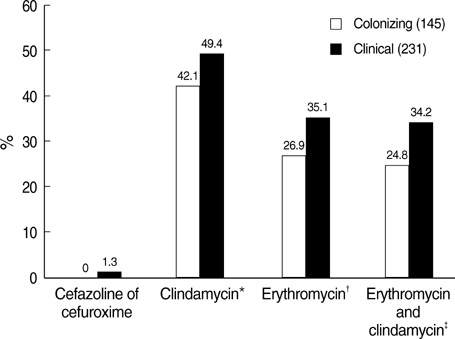
- The prevalence of group B streptococcus (GBS) among pregnant women and disease burdens in neonates and adults are increasing in Korea. Colonizing isolates, collected by screening pregnant women (n=196), and clinical isolates collected from clinical patients throughout Korea (n=234), were serotyped and screened for antibiotic resistance. Serotype III (29.8%) and V (27.7%) predominated, followed by Ia (17.0%). Antibiotic resistance was higher among clinical than colonizing isolates for erythromycin (35.1% and 26.9%; P=0.10) and for clindamycin (49.4% and 42.1%; P=0.17). erm(B) occurred in 91.9% of erythromycin resistant isolates, and 84.0% of isolates resistant to clindamycin. Only five isolates (4.2%) resistant to erythromycin were susceptible to clindamycin; by contrast, and unique to Korea, 34% of isolates resistant to clindamycin were erythromycin susceptible. Among these 60 erythromycin-susceptible & clindamycin-resistant isolates, 88% was serotype III, and lnu(B) was found in 89% of strains. Four fifths of the serotype V isolates were resistant to both erythromycin and clindamycin. Further characterization of the genetic assembly of these resistance conferring genes, erm(B) and lnu(B), will be useful to establish the clonal lineages of multiple resistance genes carrying strains.
Figure
Cited by 7 articles
-
Clonal Distribution of Clindamycin-Resistant Erythromycin-Susceptible (CRES)
Streptococcus agalactiae in Korea Based on Whole Genome Sequences
Takashi Takahashi, Takahiro Maeda, Seungjun Lee, Dong-Hyun Lee, Sunjoo Kim
Ann Lab Med. 2020;40(5):370-381. doi: 10.3343/alm.2020.40.5.370.Clinical Practice Guidelines for Soft Tissue Infections
, , , ,
Infect Chemother. 2012;44(4):213-232. doi: 10.3947/ic.2012.44.4.213.Risk Factors Associated with Group B Streptococcus Resistant to Clindamycin and Erythromycin in Pregnant Korean Women
Ji-Hyoung Yook, Moon Young Kim, Eun Ju Kim, Jae Hyug Yang, Hyun-Mee Ryu, Kwan Young Oh, Jung-Hwan Shin, Betsy Foxman, Moran Ki
Infect Chemother. 2013;45(3):299-307. doi: 10.3947/ic.2013.45.3.299.Group B Streptococcal Disease in Korean Neonates
Chi Eun Oh
Korean J Pediatr Infect Dis. 2012;19(2):43-54. doi: 10.14776/kjpid.2012.19.2.43.Group B Streptococcal Disease in Korean Neonates
Chi Eun Oh
Korean J Pediatr Infect Dis. 2012;19(2):43-54. doi: 10.14776/kjpid.2012.19.2.43.Evaluation of the Early Onset Neonatal Sepsis according to Two Antenatal Group B Streptococcus Screening Methods: Risk-Based versus Universal Screening
Jee Youn Hong, Soo Hyun Kim, Seon Mi Kim, Cheong A Yee, Suk-Joo Choi, Soo-young Oh, Cheong-Rae Roh
Perinatology. 2019;30(4):200-207. doi: 10.14734/PN.2019.30.4.200.Group B Streptococcus Colonization, Antibiotic Susceptibility, and Serotype Distribution among Saudi Pregnant Women
Amr Mohamed Mohamed, Mubashir Ahmad Khan, Aftab Faiz, Jawwad Ahmad, Elsheikh Babiker Khidir, Mohammed Abubakar Basalamah, Akhmed Aslam
Infect Chemother. 2020;52(1):70-81. doi: 10.3947/ic.2020.52.1.70.
Reference
-
1. Fry RM. Fatal infections by haemolytic streptococcus group B. Lancet. 1933. 1:199–201.2. Baker CJ, Barrett FF, Gordon RC, Yow MD. Suppurative meningitis due to streptococci of Lancefield group B: a study of 33 infants. J Pediatr. 1973. 82:724–729.
Article3. Farley MM, Harvey RC, Stull T, Smith JD, Schuchat A, Wenger JD, Stephens DS. A population-based assessment of invasive disease due to group B streptococcus in nonpregnant adults. N Engl J Med. 1993. 328:1807–1811.
Article4. Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, Stefonek KR, Albanese BA, Zell ER, Schuchat A, Schrag SJ. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008. 299:2056–2065.5. Maione D, Margarit I, Rinaudo CD, Masignani V, Mora M, Scarselli M, Tettelin H, Brettoni C, Iacobini ET, Rosini R, D'Agostino N, Miorin L, Buccato S, Mariani M, Galli G, Nogarotto R, Nardi Dei V, Vegni F, Fraser C, Mancuso G, Teti G, Madoff LC, Paoletti LC, Rappuoli R, Kasper DL, Telford JL, Grandi G. Identification of a universal Group B streptococcus vaccine by multiple genome screen. Science. 2005. 309:148–150.
Article6. Slotved HC, Kong F, Lambertsen L, Sauer S, Gilbert GL. Serotype IX, a proposed new Streptococcus agalactiae serotype. J Clin Microbiol. 2007. 45:2929–2936.
Article7. Lee HJ, Cho HK, Kim KH, Oh SH, Cha SH, Hur JK. Invasive bacterial infection in immunocompetent children in Korea: a retrospective multicenter study. 2007. In : 5th World Congress of the World Society for Pediatric Infectious Diseases-WSPID; Bangkok, Thailand. 112.8. Gygax SE, Schuyler JA, Kimmel LE, Trama JP, Mordechai E, Adelson ME. Erythromycin and clindamycin resistance in group B streptococcal clinical isolates. Antimicrob Agents Chemother. 2006. 50:1875–1877.
Article9. Brimil N, Barthell E, Heindrichs U, Kuhn M, Lutticken R, Spellerberg B. Epidemiology of Streptococcus agalactiae colonization in Germany. Int J Med Microbiol. 2006. 296:39–44.
Article10. Schrag SJ, Whitney CG, Schuchat A. Neonatal group B streptococcal disease: how infection control teams can contribute to prevention efforts. Infect Control Hosp Epidemiol. 2000. 21:473–483.11. Lee BK, Song YR, Kim MY, Yang JH, Shin JH, Seo YS, Oh KY, Yoon HR, Pai SY, Foxman B, Ki M. Epidemiology of group B streptococcus in Korean pregnant women. Epidemiol Infect. 2010. 138:292–298.
Article12. NCCLS. Performance Standard for Antimicrobial Susceptibility Testing, M100-S12. Table 2H. 2002. Wayne, PA, USA: NCCLS.13. Borchardt SM, Foxman B, Chaffin DO, Rubens CE, Tallman PA, Manning SD, Baker CJ, Marrs CF. Comparison of DNA dot blot hybridization and lancefield capillary precipitin methods for group B streptococcal capsular typing. J Clin Microbiol. 2004. 42:146–150.
Article14. Zhang L, Srinivasan U, Marrs CF, Ghosh D, Gilsdorf JR, Foxman B. Library on a slide for bacterial comparative genomics. BMC Microbiol. 2004. 4:12.15. Harris TO, Shelver DW, Bohnsack JF, Rubens CE. A novel streptococcal surface protease promotes virulence, resistance to opsonophagocytosis, and cleavage of human fibrinogen. J Clin Invest. 2003. 111:61–70.
Article16. Zhang L, Reddi U, Srinivasan U, Li S, Borchardt SM, Pillai P, Mehta P, Styka AN, Debusscher J, Marrs CF, Foxman B. Combining microarray technology and molecular epidemiology to identify genes associated with invasive group B streptococcus. Interdiscip Perspect Infect Dis. 2008. 314762.17. Marrs CF, Zhang L, Tallman P, Manning SD, Somsel P, Raz P, Colodner R, Jantunen ME, Siitonen A, Saxen H, Foxman B. Variations in 10 putative uropathogen virulence genes among urinary, faecal and peri-urethral Escherichia coli. J Med Microbiol. 2002. 51:138–142.
Article18. Lee K, Shin JW, Chong Y, Mikamo H. Trends in serotypes and antimicrobial susceptibility of group B streptococci isolated in Korea. J Infect Chemother. 2000. 6:93–97.19. Uh Y, Kim HY, Jang IH, Hwang GY, Yoon KJ. Correlation of serotypes and genotypes of macrolide-resistant Streptococcus agalactiae. Yonsei Med J. 2005. 46:480–483.20. Desjardins M, Delgaty KL, Ramotar K, Seetaram C, Toye B. Prevalence and mechanisms of erythromycin resistance in group A and group B streptococcus: implications for reporting susceptibility results. J Clin Microbiol. 2004. 42:5620–5623.
Article21. Schoening TE, Wagner J, Arvand M. Prevalence of erythromycin and clindamycin resistance among Streptococcus agalactiae isolates in Germany. Clin Microbiol Infect. 2005. 11:579–582.
Article22. Kim MW, Jang HO, Chang DY, Cho JR, Kim YA, Choi HM, Kim SH, Lee JP, Hwang KJ, Kang BH. Group B streptococcal colonization rate in Korean pregnant women. Korean J Obstet Gynecol. 2006. 49:337–344.23. Leclercq R, Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991. 35:1267–1272.
Article24. Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996. 40:1817–1824.
Article25. Kataja J, Huovinen P, Skurnik M, Seppala H. Erythromycin resistance genes in group A streptococci in Finland. The Finnish Study Group for Antimicrobial Resistance. Antimicrob Agents Chemother. 1999. 43:48–52.26. de Azavedo JC, McGavin M, Duncan C, Low DE, McGeer A. Prevalence and mechanisms of macrolide resistance in invasive and noninvasive group B streptococcus isolates from Ontario, Canada. Antimicrob Agents Chemother. 2001. 45:3504–3508.
Article27. Hsueh PR, Teng LJ, Lee LN, Ho SW, Yang PC, Luh KT. High incidence of erythromycin resistance among clinical isolates of Streptococcus agalactiae in Taiwan. Antimicrob Agents Chemother. 2001. 45:3205–3208.
Article28. Bozdogan B, Berrezouga L, Kuo M-S, Yurek DA, Farley KA, Stockman BJ, Leclercq R. A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in enterococcus faecium HM1025. Antimicrob Agents Chemother. 1999. 43:925–929.
Article29. Varaldo PE, Montanari MP, Giovanetti E. Genetic elements responsible for erythromycin resistance in streptococci. Antimicrob Agents Chemother. 2009. 53:343–353.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Manifestation Patterns and Trends in Poststreptococcal Glomerulonephritis
- Molecular Epidemiologic Study of Streptococcus pyogenes Analyzed by T protein Serotyping and Pulsed Field Gel Elecrophoresis(PFGE) in Normal Children
- Antibiotic-Resistant Streptococcus pneumoniae
- The Changing Epidemiology of Childhood Pneumococcal Disease in Korea
- A Case of Late Onset Neonatal Bacteremia and Meningitis Caused by Streptococcus lutetiensis