J Korean Ophthalmol Soc.
2014 May;55(5):693-701. 10.3341/jkos.2014.55.5.693.
Changes in the Retinal Nerve Fiber Layer after Intravitreal Injections of Bevacizumab in Glaucoma Patients
- Affiliations
-
- 1Department of Ophthalmology and Visual Science, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. ckpark@catholic.ac.kr
- KMID: 2218090
- DOI: http://doi.org/10.3341/jkos.2014.55.5.693
Abstract
- PURPOSE
To examine retinal nerve fiber layer (RNFL) changes after intravitreal injection of bevacizumab in patients with or without underlying glaucoma.
METHODS
A total of 104 eyes of 104 patients with retinal disease undergoing intravitreal injection of bevacizumab were prospectively investigated. Bevacizumab injections (1.25/0.05 mg/mL) were performed using a standardized technique. In the patient who had pretreatment with intraocular pressure (IOP)-lowering medication, 1 drop of brimonidine was instilled 30 minutes before the injection. Before and after the intravitreal injections, the patients were monitored for IOP and evaluated with optical coherence tomography using Stratus at least 3 months after the injection.
RESULTS
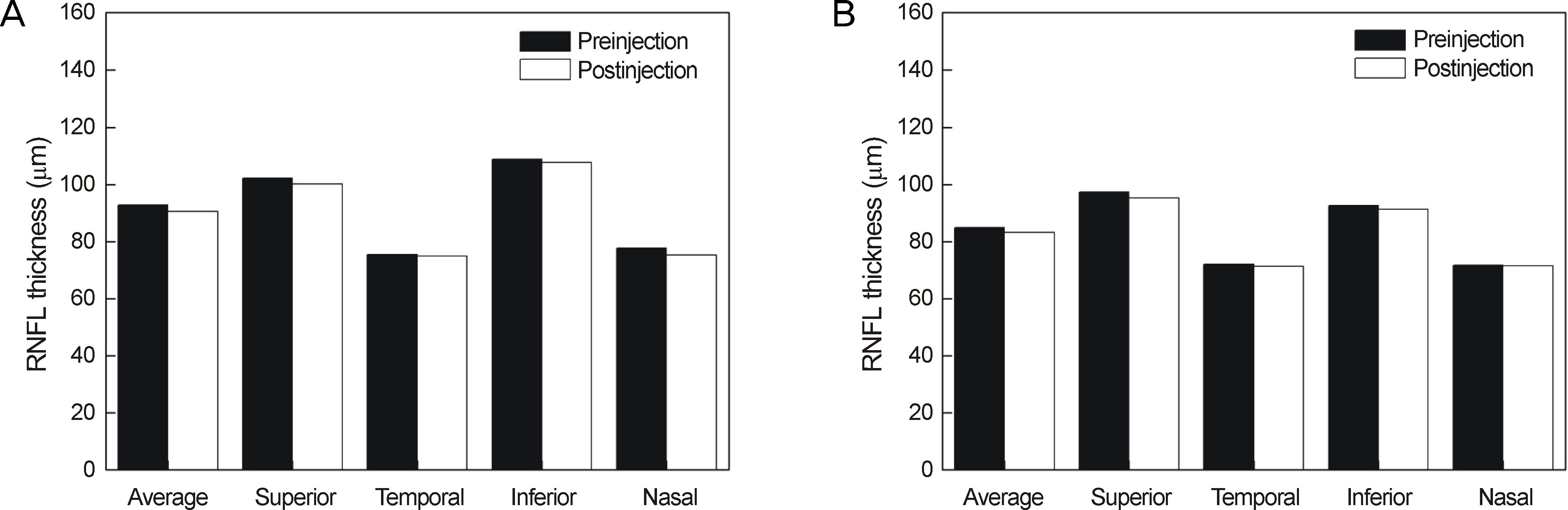
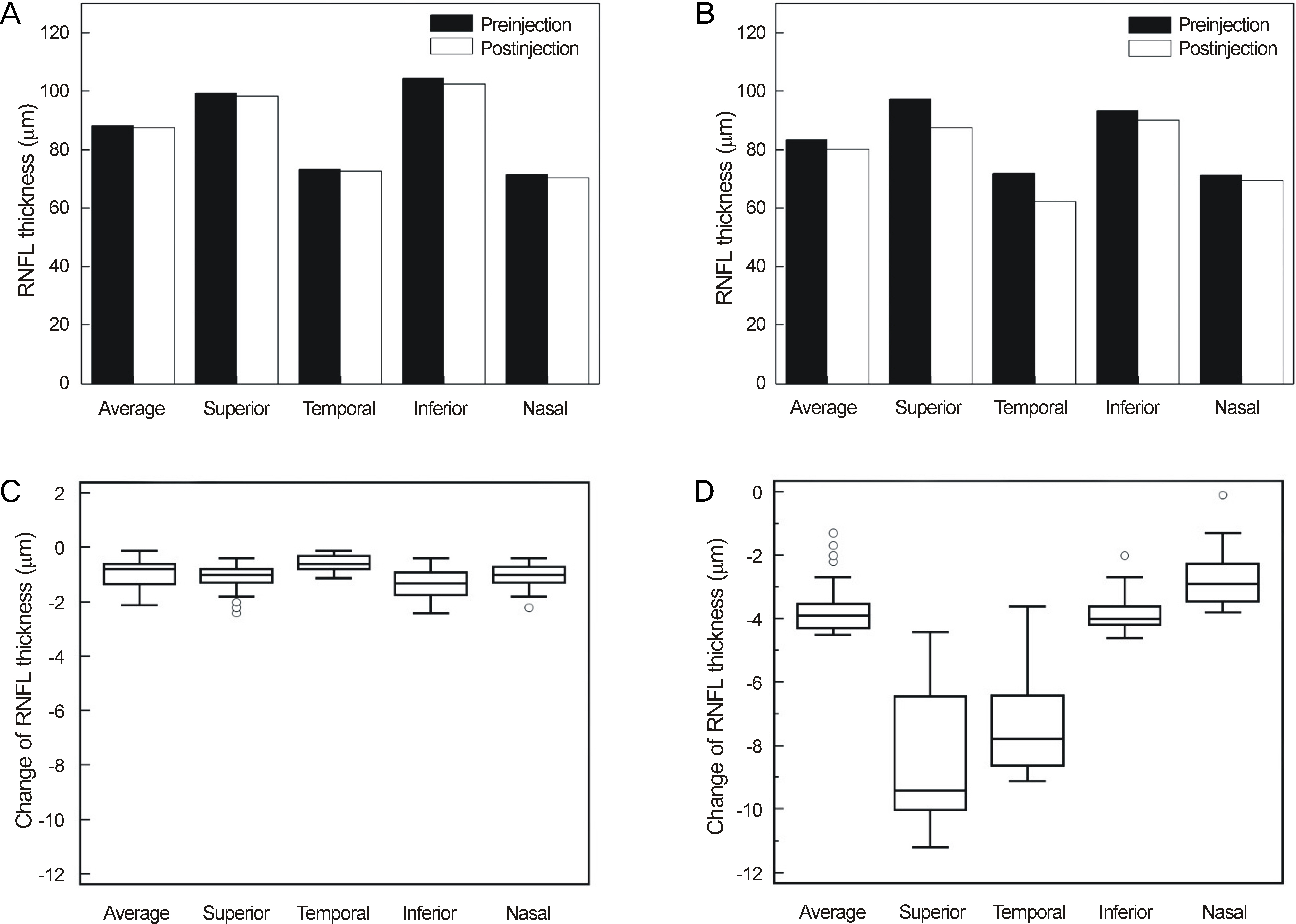
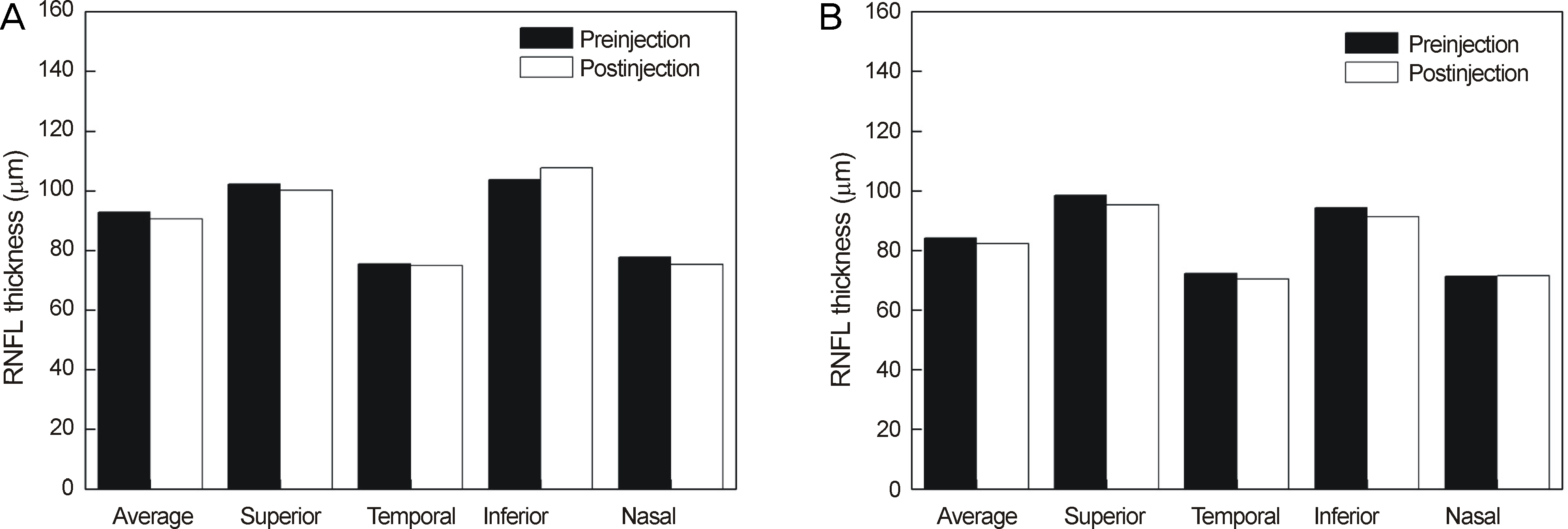
Thirty minutes after injection, 6.4% of patients had an IOP over 30 mm Hg in the non-pretreatment group while no patient had an IOP over 30 mm Hg in the pretreatment group. In eyes with only retinal diseases, the RNFL thickness did not change significantly after the injection regardless of pretreatment, whereas in eyes with underlying glaucomatous damage and no pretreatment, significant decrease in RNFL thickness was observed at the superior (p = 0.036) and temporal (p = 0.048) sectors of the optic nerve head without pretreatment.
CONCLUSIONS
Intravitreal injection of bevacizumab did not typically cause significant changes in RNFL thickness; however, in eyes with underlying glaucoma without pretreatment, a significant decrease in RNFL thickness was observed in the superior and temporal sectors of the optic nerve head. Therefore, applying IOP-lowering pretreatment medication before intravitreal injection of bevacizumab is required for protection of RNFL in glaucoma patients.
MeSH Terms
Figure
Cited by 1 articles
-
Ganglion Cell Layer Thickness after Anti-Vascular Endothelial Growth Factor Treatment in Retinal Vein Occlusion
Ji Young Lee, Hyung Chan Kim
J Korean Ophthalmol Soc. 2016;57(1):63-70. doi: 10.3341/jkos.2016.57.1.63.
Reference
-
References
1. Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004; 26:943–54.
Article2. Zachary I. Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biologic function, and therapeutic potential. Neurosignals. 2005; 14:207–21.3. Lee SJ, Koh HJ. Enlargement of the foveal avascular zone in diabetic retinopathy after adjunctive intravitreal bevacizumab (Avasin) with pars plana vitrectomy. J Ocul Pharmacol Ther. 2009; 25:173–4.4. Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol. 2008; 19:927–34.
Article5. Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006; 90:1344–9.
Article6. Wu L, Martínez-Castellanos MA, Quiroz-Mercado H, et al. Twelvemonth safety of intravitreal injections of bevacizumab (Avastin): results of the PanAmerican Collaborative Retina Study Group (PACORES). Graefes Arch Clin Exp Ophthalmol. 2008; 246:81–7.
Article7. Chung EJ, Hong YT, Lee SC, et al. Prognostic factors for visual outcome after intravitreal bevacizumab for macular edema due to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2008; 246:1241–7.
Article8. Chung EJ, Roh MI, Kwon OW, Koh HJ. Effects of macular ischemia on the outcome of intravitreal bevacizumab therapy for diabetic macular edema. Retina. 2008; 28:957–63.
Article9. Falkenstein IA, Cheng L, Freeman WR. Changes of intraocular pressure after intravitreal injection of bevacizumab (avastin). Retina. 2007; 27:1044–7.
Article10. Hollands H, Wong J, Bruen R, et al. Short-term intraocular pressure changes after intravitreal injection of bevacizumab. Can J Ophthalmol. 2007; 42:807–11.
Article11. Lee K, Yang H, Lim H, Lew HM. A prospective study of blood pressure and intraocular pressure changes in hypertensive and nonhypertensive patients after intravitreal bevacizumab injection. Retina. 2009; 29:1409–17.
Article12. Bakri SJ, McCannel CA, Edwards AO, Moshfeghi DM. Persisent ocular hypertension following intravitreal ranibizumab. Graefes Arch Clin Exp Ophthalmol. 2008; 246:955–8.
Article13. Jalil A, Fenerty C, Charles S. Intravitreale bevacizumab (Avastin) causing acute glaucoma: An unreported complication. Eye (Lond). 2007; 21:1541.14. Kahook MY, Kimura AE, Wong LJ, et al. Sustained elevation in intraocular pressure associated with intravitreal bevacizumab injections. Ophthalmic Surg Lasers Imaging. 2009; 40:293–5.
Article15. Seth RK, Salim S, Shields MB, Adelman RA. Assessment of optic nerve cup-to-disk ratio changes in patients receiving multiple intravitreal injections of antivascular endothelial growth factor agents. Retina. 2009; 29:956–9.
Article16. Wu H, Chan TC. The effects of intravitreal ophthalmic medications on intraocular pressure. Semin Ophthalmology. 2009; 24:100–5.
Article17. Benz MS, Albini TA, Holz ER, et al. Short-term course of intraocular pressure after intravitreal injection of triamcinolone acetonide. Ophthalmology. 2006; 113:1174–8.
Article18. The Brimonidine-ALT Study Group. Effect of brimonidine 0.5% on intraocular pressure spikes following 360% argon laser trabeculoplasty. The Brimonidine-ALT Study Group. Ophthalmic Surg Lasers. 1995; 26:404–9.19. Barnes SD, Campagna JA, Dirks MS, Doe EA. Control of intraocular pressure elevations after argon laser trabeculoplasty: comparison of brimonidine 0.2% to apraclonidine 1.0%. Ophthalmology. 1999; 106:2033–7.20. Mordenti J, Cuthbertson RA, Ferrara N, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999; 27:536–44.
Article21. Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004; 7:335–45.
Article22. Heinzerling JH, Huerta S. Bowel perforation from bevacizumab for the treatment of metastatic colon cancer: incidence, etiology, and management. Curr Surg. 2006; 63:334–7.
Article23. Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007; 99:1232–9.
Article24. Shah MA, Ilson D, Kelsen DP. Thromboembolic events in gastric cancer: high incidence in patients receiving irinotecan-and bevacizumab-based therapy. J Clin Oncol. 2005; 23:2574–6.25. Horsely MB, Mandava N, Maycotte MA, Kahook MY. Retinal nerve fiber layer thickness in patients receiving chronic anti-vascular endothelial growth factor therapy. Am J Ophthalmol. 2010; 150:558–61.26. Seibold LK, Mandava N, Kahook MY. Comparison of retinal nerve fiber layer thickness in normal eyes using time-domain and spectral-domain optical coherence tomography. Am J Ophthalmol. 2010; 150:807–14.
Article27. Paunescu LA, Schuman JS, Price LL. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using Stratus OCT. Invest Ophthalmol Vis Sci. 2004; 45:1716–24.28. Budenz DL, Fredette MJ, Feuer WJ, Anderson DR. Reproducibility of peripapillary retinal nerve fiber thickness measurements with stratus OCT in glaucomatous eyes. Ophthalmology. 2008; 115:661–6.
Article29. Menke MN, Salam A, Framme C, Wolf S. Long-term intraocular pressure changes in patients with neovascular age-related macular degeneration treated with ranibizumab. Ophthalmologica. 2013; 229:168–72.
Article30. Knip MM, Valimaki J. Effects of pegaptanib injections on intraocular pressure with and without anterior chamber paracentesis: A Prospective Study. Acta Ophthalmol. 2012; 90:254–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in the Retinal Nerve Fiber Layer after Intravitreal Injections of Bevacizumab in Glaucoma Patients
- Reproducibility of Retinal Nerve Fiber Layer Thickness Evaluation by Nerve Fiber Analyzer
- Biometry of Retinal Nerve Fiber Layer Thickness by NFA
- The Effect of Bevacizumab on Retinal Vessel Diameter, Intraocular Pressure, Retinal Nerve Fiber Layer and the Optic Disc
- Influence of Diabetes Mellitus on the Retinal Ne rve Fiber Layer Thickness Measurement by Nerve Fiber Analyzer