J Korean Ophthalmol Soc.
2011 Jul;52(7):838-845. 10.3341/jkos.2011.52.7.838.
Intravitreal Ranibizumab Therapy for Neovascular Age-Related Macular Degeneration with a Predominantly Hemorrhagic Lesion
- Affiliations
-
- 1Department of Ophthalmology, Kang Dong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. sungpyo@hanafos.com
- KMID: 2214709
- DOI: http://doi.org/10.3341/jkos.2011.52.7.838
Abstract
- PURPOSE
To report the efficacy and safety of intravitreal ranibizumab monotherapy in patients with age-related macular degeneration with a predominantly hemorrhagic lesion.
METHODS
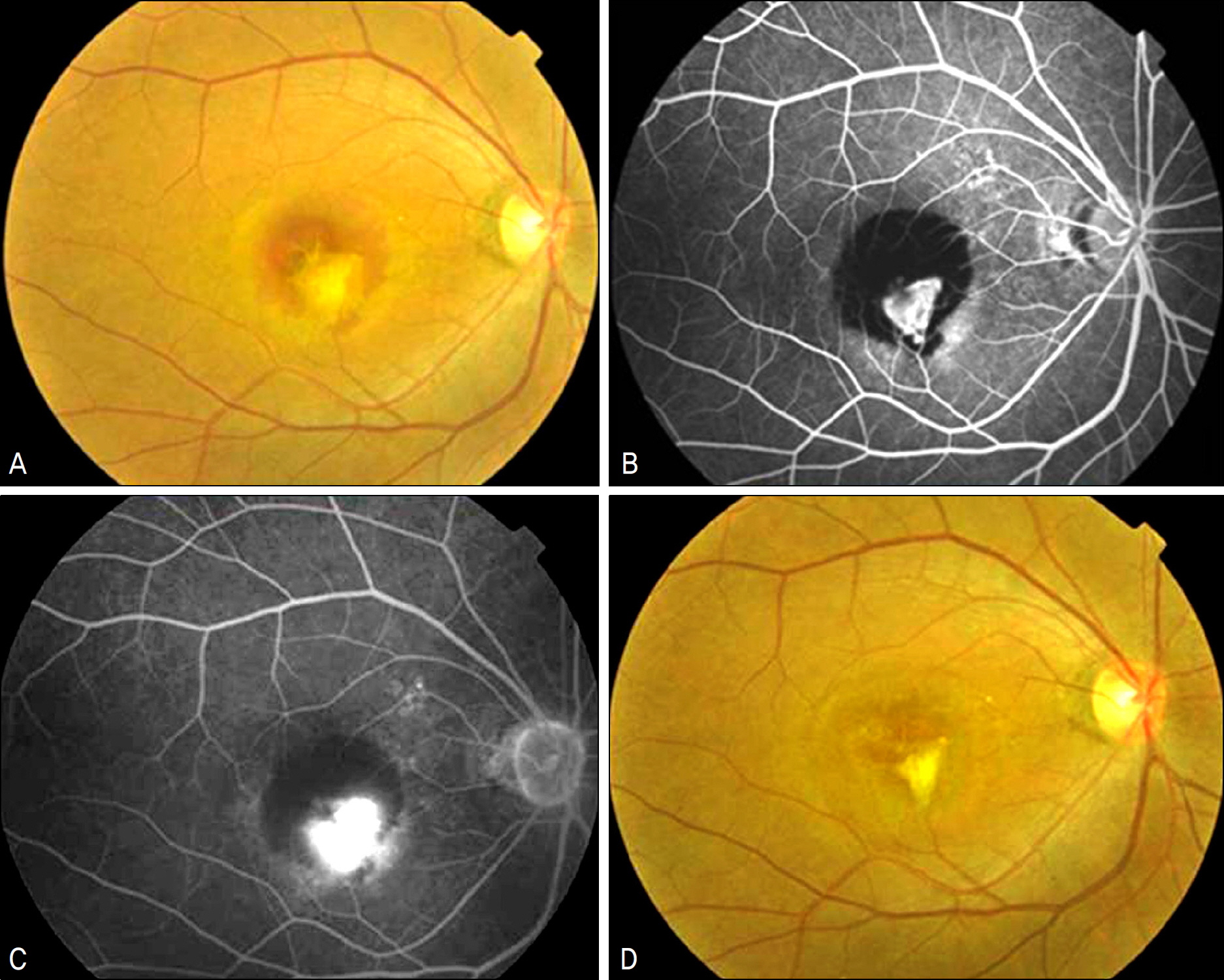
Nineteen eyes of 19 patients with submacular hemorrhage involving the fovea were treated with intravitreal ranibizumab (0.5 mg/0.05 ml) injections as needed. All patients completed at least 4 months of follow-up. Ophthalmologic examinations, fluorescence angiographic evaluations, optical coherence tomography (OCT) examination, and hemorrhage size were analyzed before the injections, and at 1,2,4,6, and 12 months follow-up.
RESULTS
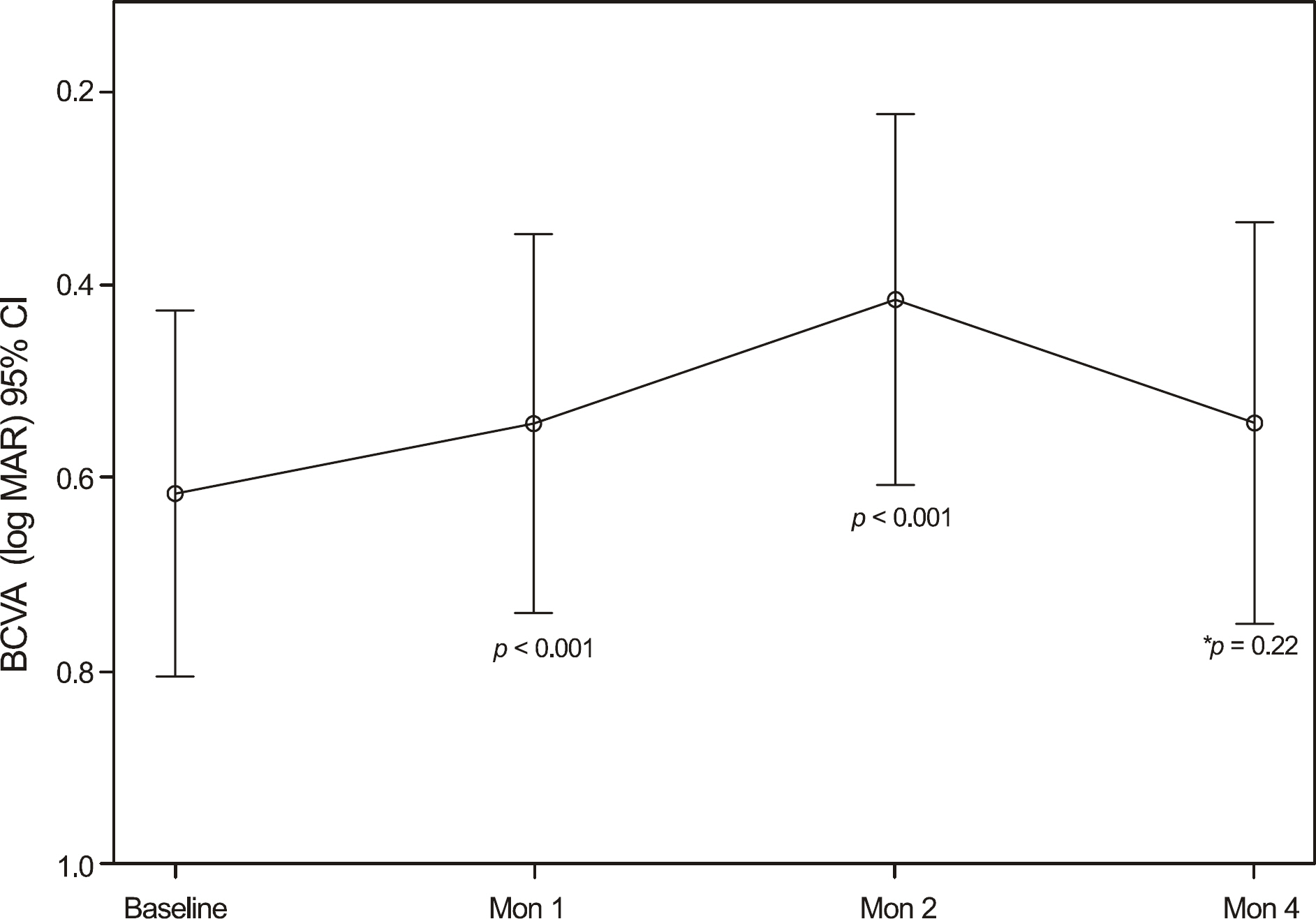
The average VA and mean central retinal thickness (CRT) before ranibizumab treatment were 0.62 +/- 0.39 log MAR and 335.76 +/- 111.22 microm, respectively. Additionally, the VA and CRT four months after the initial injections were 0.54 +/- 0.43 log MAR and 241.42 +/- 107.55 microm, respectively. The mean size of the hemorrhage was significantly reduced from 2.87 +/- 2.44 DA (disk areas) at baseline to 0.9 +/- 1.28 DA at four month follow up.
CONCLUSIONS
Intravitreal injection of ranibizumab is an effective treatment option for patients with age-related macular degeneration with a predominantly hemorrhagic lesion.
MeSH Terms
Figure
Reference
-
References
1. Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009; 116:57–65.
Article2. Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996; 16:183–9.
Article3. Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990; 109:33–7.
Article4. Berrocal MH, Lewis ML, Flynn HW Jr. Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol. 1996; 122:486–93.
Article5. Sanders D, Peyman GA, Fishman G, et al. The toxicity of intravitreal whole blood and hemoglobin. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975; 197:255–67.
Article6. Toth CA, Morse LS, Hjelmeland LM, Landers MB 3rd. Fibrin di-rects early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol. 1991; 109:723–9.
Article7. Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol. 1982; 94:762–73.
Article8. Hewitt AT, Adler R. The retinal pigment epithelium and interphotoreceptor matrix: structure and specialized functions. Ryan SJ, editor. Retina. St. Louise: CV Mosby;1994. 1:p. 58–71.9. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials–TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol. 1999; 117:1329–45.10. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.
Article11. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.
Article12. Lewis H. Intraoperative fibrinolysis of submacular hemorrhage with tissue plasminogen activator and surgical drainage. Am J Ophthalmol. 1994; 118:559–68.
Article13. Lim JI, Drews-Botsch C, Sternberg P Jr, et al. Submacular hemorrhage removal. Ophthalmology. 1995; 102:1393–9.
Article14. Ibanez HE, Williams DF, Thomas MA, et al. Surgical management of submacular hemorrhage. A series of 47 consecutive cases. Arch Ophthalmol. 1995; 113:62–9.15. Hassan AS, Johnson MW, Schneiderman TE, et al. Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology. 1999; 106:1900–6. discussion 1906–7.16. Lincoff H, Kreissig I. Intravitreal injection of tissue plasminogen activator and gas in subretinal hemorrhage caused by age-related macular degeneration. Retina. 2001; 21:191.
Article17. Cleary CA, Jungkim S, Ravikumar K, et al. Intravitreal bevacizumab in the treatment of neovascular age-related macular degeneration, 6- and 9-month results. Eye. 2008; 22:82–6.
Article18. Stifter E, Michels S, Prager F, et al. Intravitreal bevacizumab therapy for neovascular age-related macular degeneration with large submacular hemorrhage. Am J Ophthalmol. 2007; 144:886–92.
Article19. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–83.
Article20. Brown MM, Brown GC, Sharma S, et al. The burden of age-related macular degeneration: a value-based analysis. Curr Opin Ophthalmol. 2006; 17:257–66.21. Schouten JS, La Heij EC, Webers CA, et al. A systematic review on the effect of bevacizumab in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2009; 247:1–11.
Article22. Kliffen M, Sharma HS, Mooy CM, Kerkvliet S. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997; 81:154–62.
Article23. Rosenfeld PJ, Rich RM, Lalwani GA. Ranibizumab: Phase III clinical trial results. Ophthalmol Clin North Am. 2006; 19:361–72.24. Sacu S, Stifter E, Vécsei-Marlovits PV, et al. Management of extensive subfoveal haemorrhage secondary to neovascular age-related macular degeneration. Eye. 2009; 23:1404–10.
Article25. Meyer CH, Scholl HP, Eter N, et al. Combined treatment of acute subretinal haemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: a retrospective pilot study. Acta Ophthalmol. 2008; 86:490–4.
Article26. Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica. 1999; 213:97–102.
Article27. Bressler NM, Bressler SB, Childs AL, et al. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: ophthalmic findings: SST report no. 13. Ophthalmology. 2004; 111:1993–2006.28. Arias L, Monés J. Transconjunctival sutureless vitrectomy with tissue plasminogen activator, gas and intravitreal bevacizumab in the management of predominantly hemorrhagic age-related macular degeneration. Clin Ophthalmol. 2010; 4:67–72.
Article29. Rosa RH Jr, Davis JL, Eifrig CW. Clinicopathologic reports, case reports, and small case series: clinicopathologic correlation of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 2002; 120:502–8.30. Tong JP, Chan WM, Liu DT, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006; 141:456–62.
Article31. Matsuoka M, Ogata N, Otsuji T, et al. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004; 88:809–15.
Article32. Hikichi T, Ohtsuka H, Higuchi M, et al. Improvement of angiographic findings of polypoidal choroidal vasculopathy after intravitreal injection of ranibizumab monthly for 3 months. Am J Ophthalmol. 2010; 150:674–82.
Article33. Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol. 2002; 133:639–48.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravitreal Ranibizumab Therapy for Neovascular Age-Related Macular Degeneration with a Predominantly Hemorrhagic Lesion
- Intravitreal Aflibercept for Neovascular Age-Related Macular Degeneration Resistant to Bevacizumab and Ranibizumab
- Treatment of Exudative Age-Related Macular Degeneration
- Efficacy of Three Aflibercept Injections for Neovascular Age-related Macular Degeneration Showing Limited Response to Ranibizumab
- Comparison between Aflibercept, Ranibizumab Intravitreal Injection on Neovascular Age-related Macular Degeneration Patients