J Korean Ophthalmol Soc.
2009 May;50(5):725-730. 10.3341/jkos.2009.50.5.725.
The Efficacy of Ranibizumab for Choroidal Neovascularization in Age-related Macular Degeneration
- Affiliations
-
- 1Department of Ophthalmology and Visual Science, College of Medicine, The Catholic University of Korea, Seoul, Korea. youngjungroh@hanmail.net
- KMID: 2212308
- DOI: http://doi.org/10.3341/jkos.2009.50.5.725
Abstract
-
PURPOSE: To report 1-year clinical changes in visual acuity (VA) after intravitreal ranibizumab therapy for choroidal neovascularization (CNV) due to age-related macular degeneration (AMD), and to determine differences in treatment effects according to the CNV subtype.
METHODS
Forty six patients (46 eyes) with subfoveal CNV were treated with intravitreal ranibizumab (0.5 mg) injections as needed. Visual acuity, fluorescein angiography, and macular OCT were examined after 12 months. The patients were divided into two groups: Classic CNV and occult CNV. The VA of the two groups was compared 12 months after the initial injections.
RESULTS
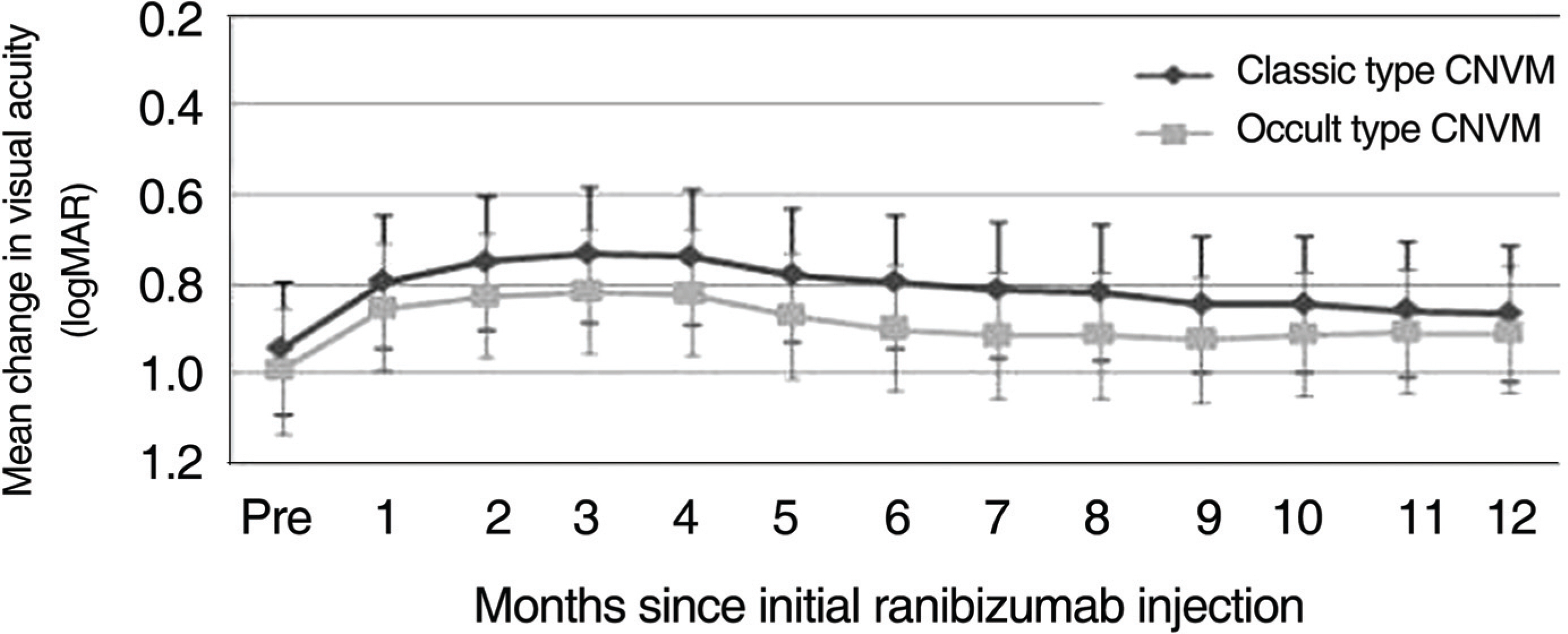
The average VA and mean central retinal thickness (CRT) before ranibizumab treatment was 1.011+/-0.408 logMAR and 335.3 microm, respectively, and the VA and mean CRT 12 months after the initial injections was 0.928+/-0.357 logMAR and 246.2 microm, respectively (p=0.042, p<0.001). Out of 46 eyes, 13 eyes (28.3%) had a VA that improved by more than 0.1 logMAR, 33 eyes (71.7%) had VA that changed less than 0.1 logMAR, and 40 eyes (86.6%) had a VA that changed by less than 0.3 logMAR. The VA improved to 0.084 logMAR in classic CNV (18 eyes) and to 0.081 logMAR in occult CNV (28 eyes) after 12 months, though the difference between groups was not significant (p=0.910). CONCLUSIONS: Intravitreal injection of ranibizumab is an effective treatment for patients with subfoveal CNV secondary to AMD to improve or stabilize VA, and the effect of treatment on VA is not significantly different according to the CNV subtype.
MeSH Terms
Figure
Cited by 2 articles
-
Long-Term Effect of Intravitreal Ranibizumab Injection on Choroidal Neovascularization in Age-Related Macular Degeneration
Hyo Ju Jang, Su Jeong Song, Jeong Hoon Bae
J Korean Ophthalmol Soc. 2013;54(9):1359-1364. doi: 10.3341/jkos.2013.54.9.1359.Efficacy of Three Aflibercept Injections for Neovascular Age-related Macular Degeneration Showing Limited Response to Ranibizumab
Kyung Min Kim, Jae Hui Kim, Young Suk Chang, Jong Woo Kim, Chul Gu Kim, Dong Won Lee
J Korean Ophthalmol Soc. 2017;58(1):62-68. doi: 10.3341/jkos.2017.58.1.62.
Reference
-
References
1. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Stydy. Ophthalmology. 1992; 99:933–43.2. Ferris FL, Fine SL, Hyman LA. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984; 107:1640–2.
Article3. Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005; 25:111–8.
Article4. Ng EW, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005; 40:352–68.
Article5. Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007; 144:850–7.
Article6. Gragoudas ES, Adamis AP, Cunningham ET, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004; 351:2805–16.
Article7. Cleary CA, Jungkim S, Ravikumar K, et al. Intravitreal bevacizumab in the treatment of neovascular age-related macular degeneration, 6 and 9 month results. Eye. 2008; 22:82–6.8. Ferrara N, Damica L, Shams N, et al. Development of ranibizumab, an antivascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26:859–70.
Article9. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006; 355:1419–31.
Article10. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Eng J Med. 2006; 355:1432–44.
Article11. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–83.
Article12. Bashshur ZF, Haddad ZA, Schakal A, et al. Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: a one-year prospective study. Am J Ophthalmol. 2008; 145:249–56.
Article13. Hijikata K, Masuda K. Visual prognosis in Behcet's disease: effects of cyclophosphamide and colchicine. Jpn J Ophthalmol. 1978; 22:506–19.14. Penn JS, Madan A, Caldwell RB, et al. Vascular endothelial growth factor in eye disease. Prog Retina Eye Res. 2008; 27:331–71.
Article15. Treatment of age-related macular degeneration with photodynamic therapy study group, verteporfin in photodynamic therapy study group. effect of lesion size, visual acuity, and lesion composition on visual acuity change with and without verteporfin therapy for choroidal neovascularization secondary to age-related macular degeneration: TAP and VIP report no. 1. Am J Ophthalmol. 2003; 136:407–18.16. Treatment of age-related macular degeneration with photodynamic therapy (TAP) study group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials-TAP report. Arch Ophthalmol. 1999; 117:1329–45.17. Verteporfin in photodynamic therapy study group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of randomized clinical trial including lesions with occult with no classic choroidal neovascularization-verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001; 131:541–60.18. Lazic R, Gabric N. Verteporfin therapy and intravitreal bevacizumab combined and alone in choroidal neovascularization due to age-related macular degeneration. Ophthalmology. 2007; 114:1179–85.
Article19. Azad RV, Khan MA, Chanana B, Azad S. Intravitreal bevacizumab for subfoveal choroidal neovascularization secondary to age-related macular degeneration in an Indian population. Jpn J Ophthalmol. 2008; 52:52–6.
Article20. Boyer DS, Antoszyk AN, Awh CC, et al. Subgroup Analysis of the MARINA Study of Ranibizumab in Neovascular Age-Related Macular Degeneration. Ophthalmology. 2007; 114:246–52.
Article21. Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007; 144:850–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Macular Hole Following Intravitreal Ranibizumab Injections for Choroidal Neovascularization
- Incidence of New Choroidal Neovascularization in Fellow Eyes of Patients Treated for Age-Related Macular Degeneration
- Long-Term Effect of Intravitreal Ranibizumab Injection on Choroidal Neovascularization in Age-Related Macular Degeneration
- Short-term Efficacy and Safety of Ranibizumab for Neovascular Age-related Macular Degeneration in the Real World: A Post-marketing Surveillance Study
- The Efficacy of Ranibizumab for Choroidal Neovascularization in Age-related Macular Degeneration