Korean J Gastroenterol.
2012 Dec;60(6):373-376. 10.4166/kjg.2012.60.6.373.
A Case of Squamous Cell Carcinoma of the Breast in a Patient with Crohn's Disease Taking Azathioprine
- Affiliations
-
- 1Department of Internal Medicine, Catholic University of Daegu, School of Medicine, Daegu, Korea. kimey@cu.ac.kr
- 2Department of Pathology, Catholic University of Daegu, School of Medicine, Daegu, Korea.
- KMID: 1773074
- DOI: http://doi.org/10.4166/kjg.2012.60.6.373
Abstract
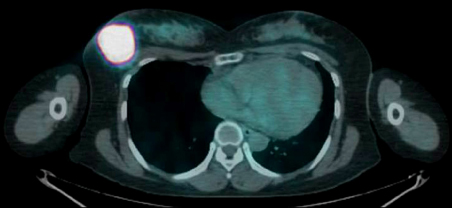
- Azathioprine (AZA) treatment in transplant or autoimmune patients and subsequent appearance squamous cell carcinomas at various sites, particularly skin and cervix, has shown a close relationship. However, it remains uncertain whether this is true for the patients with Crohn's disease. We report a case of squamous cell carcinoma of the breast occurred in a 35-year-old female with Crohn's disease taking AZA. She was first diagnosed with Crohn's disease 10 years ago and has taken AZA with 5-aminosalicylic acid (5-ASA) on regular follow up in gastrointestinal department for 9 years. She had no family history of breast cancer. She visited breast cancer clinic due to incidentally found right breast mass. A mastectomy on the right breast was performed and 6.3x5.5 cm mass was removed. The mass was microscopically proven to be poorly differentiated squamous cell carcinoma with focal keratin pearl formation. At age of 25, she was first diagnosed with active Crohn's disease. 5-ASA and corticosteroid induced remission. Then, steroid was tapered off and AZA was maintained at 1 mg/kg due to leukopenia at higher dose. She stopped taking AZA at her discretion during her two pregnancies and reported total of 67 months of AZA medication on her breast cancer diagnosis.
MeSH Terms
-
Adult
Azathioprine/*therapeutic use
Breast Neoplasms/*diagnosis/pathology/therapy
Carcinoma, Squamous Cell/*diagnosis/pathology/therapy
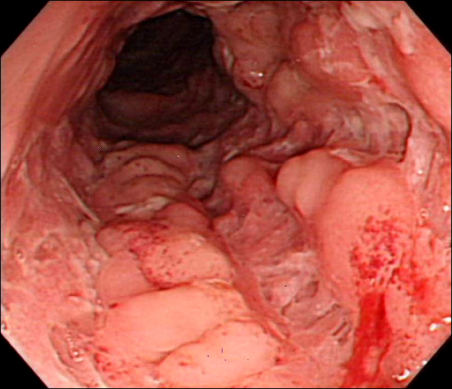
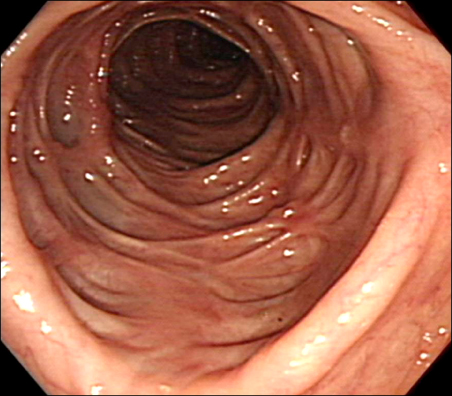
Colonoscopy
Combined Modality Therapy
Crohn Disease/*drug therapy
Female
Humans
Immunosuppressive Agents/*therapeutic use
Mesalamine/therapeutic use
Positron-Emission Tomography
Immunosuppressive Agents
Azathioprine
Mesalamine
Figure
Cited by 1 articles
-
A Case of Pleomorphic Liposarcoma in a Patient with Crohn's Disease Taking Azathioprine
Soo Min Ahn, Seong O Suh, Yu Mi Oh, Chang Yong Yun, Hyoung Hun Sim, Chae A Park, Cheol Min Song, Ji Yoon Bae
Korean J Gastroenterol. 2013;62(4):248-252. doi: 10.4166/kjg.2013.62.4.248.
Reference
-
1. Kim CG, Kim JW, Kim HD, et al. Clinical features of Crohn's disease in Korea. Korean J Gastroenterol. 2002. 40:173–180.2. Fraser AG, Orchard TR, Robinson EM, Jewell DP. Long-term risk of malignancy after treatment of inflammatory bowel disease with azathioprine. Aliment Pharmacol Ther. 2002. 16:1225–1232.3. Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005. 54:1121–1125.4. Kim WH, Cho JH, Kim TI. Rational dosing of azathioprine and 6-mercaptopurine in inflammatory bowel diseases. Korean J Gastroenterol. 2003. 41:423–437.5. Lee HJ, Yang SK, Kim KJ, et al. The safety and efficacy of Azathioprine and 6-mercaptopurine in the treatment of Korean patients with Crohn's disease. Intest Res. 2009. 7:22–31.6. Kim JH, Cheon JH, Kim WH. The frequency and the course of the adverse effects of azathioprine/6-mercaptopurine treatment in patients with inflammatory bowel disease. Korean J Gastroenterol. 2008. 51:291–297.7. Cohen SM, Erturk E, Skibba JL, Bryan GT. Azathioprine induction of lymphomas and squamous cell carcinomas in rats. Cancer Res. 1983. 43:2768–2772.8. Beaugerie L, Brousse N, Bouvier AM, et al. CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009. 374:1617–1625.9. Smith MA, Irving PM, Marinaki AM, Sanderson JD. Review article: malignancy on thiopurine treatment with special reference to inflammatory bowel disease. Aliment Pharmacol Ther. 2010. 32:119–130.10. Nair B, Sukumar S, Poolari GK, Appu T. Azathioprine-induced squamous cell carcinoma of the kidney. Scand J Urol Nephrol. 2007. 41:173–175.11. Shin US, Yu CS, Kim CW, et al. Malignancy associated with inflammatory bowel disease. J Korean Soc Coloproctol. 2009. 25:150–156.12. Samarasena J, Borgaonkar M. Development of hepatocellular carcinoma in a patient with Crohn's disease treated with azathioprine. Dig Dis Sci. 2007. 52:2748–2750.13. Das DK, Samhan M, Bashir HM, et al. Metaplastic carcinoma of the breast in a renal transplant recipient. Initial diagnosis by fine needle aspiration cytology and immunocytochemistry. Acta Cytol. 1994. 38:917–922.14. Gibson GR, Qian D, Ku JK, Lai LL. Metaplastic breast cancer: clinical features and outcomes. Am Surg. 2005. 71:725–730.15. Greenstein AJ, Gennuso R, Sachar DB, et al. Extraintestinal cancers in inflammatory bowel disease. Cancer. 1985. 56:2914–2921.16. Kim HC, Nam SW, Cho YK, et al. A case of non-Hodgkin's lymphoma in a patient with Crohn's disease. Korean J Gastroenterol. 2006. 47:233–237.17. Song IS, Choi KW, Kim JW, et al. Acute myelogenous leukemia in Crohn's disease. Korean J Gastroenterol. 1998. 31:263–268.18. Kim DS, Lee HS, Yoon JH, Lee DC, Lee SH, Kim JW. A case of malignant lymphoma in patient with ulcerative colitis. Korean J Gastrointest Endosc. 2001. 23:188–191.19. Han KR, Yu CS, Yang SK, et al. A case of non-Hodgkin's lymphoma in ulcerative colitis. J Korean Soc Coloproctol. 2005. 21:52–56.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Squamous Cell Carcinoma of the Breast in a Patient with Crohn's Disease Taking Azathioprine
- A Case of Pleomorphic Liposarcoma in a Patient with Crohn's Disease Taking Azathioprine
- Fine Needle Aspiration Cytology of Squamous Cell Carcinoma of the Breast: Report of A Case
- Azathioprine-Induced Non-Cirrhotic Portal Hypertension in a Patient with Crohn’s Disease
- Primary Squamous Cell Carcinoma of the Breast