The Incidence and Clinical Features of Clostridium difficile Infection; Single Center Study
- Affiliations
-
- 1Department of Internal Medicine, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea. yousunk69@korea.com
- 2Department of Laboratory Medicine, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- KMID: 1775899
- DOI: http://doi.org/10.4166/kjg.2010.55.3.175
Abstract
- BACKGROUND/AIMS
Clostridium difficile is the predominant cause of nosocomial diarrhea. Recently, the incidence of Clostridium difficile infection (CDI) increases in Europe and North America. A retrospective study was performed to evaluate the change of incidence and clinical features of CDI in Korea.
METHODS
From January 2003 to December 2008, inpatients diagnosed with CDI in Seoul Paik hospital were enrolled. The diagnosis of CDI was made when patients complained diarrhea with any positive results in C. difficile toxin assay, stool culture, or endoscopy. The incidence, recurrence rate, and clinical features were compared between early period (2003-2005) and late period (2006-2008).
RESULTS
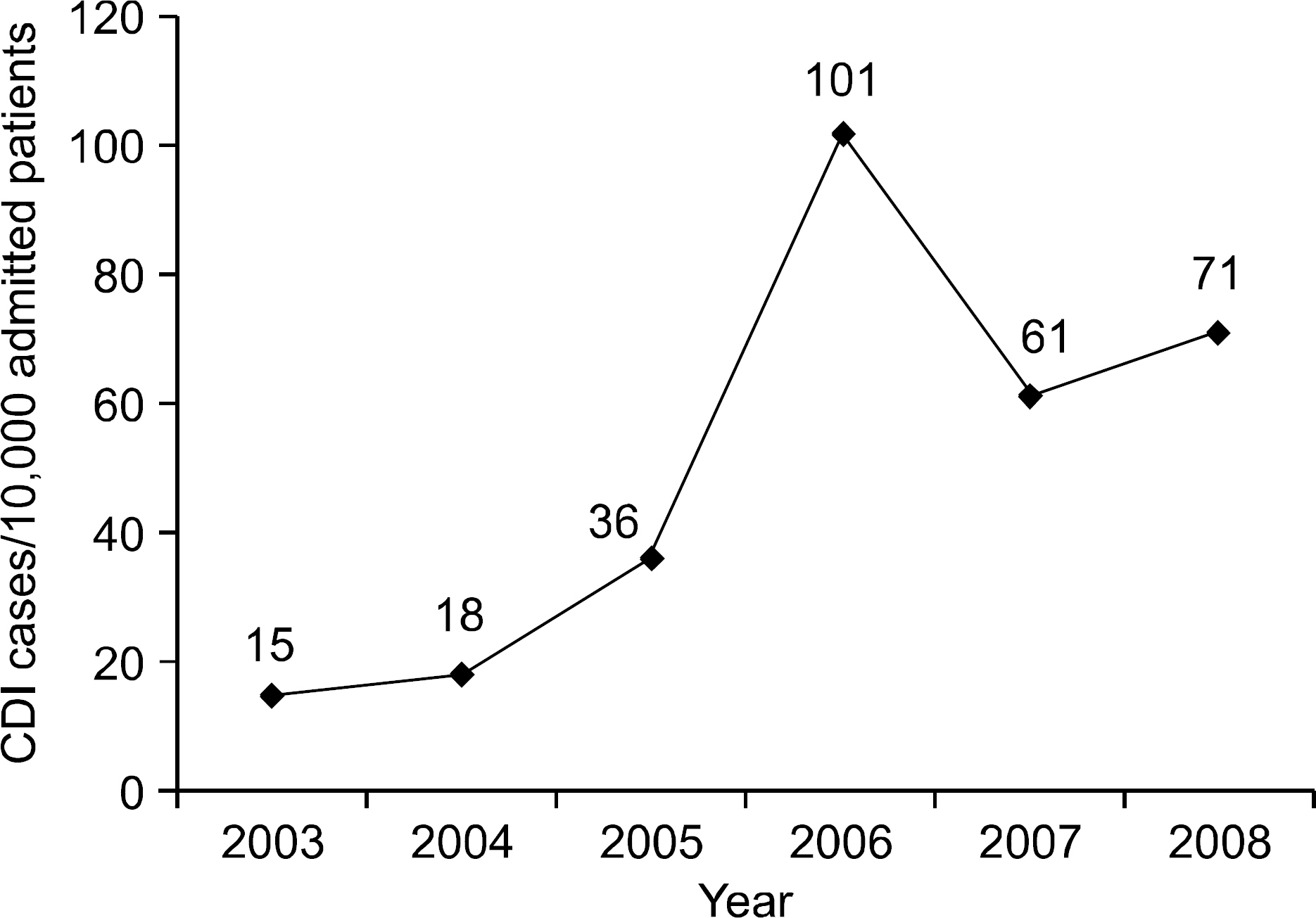
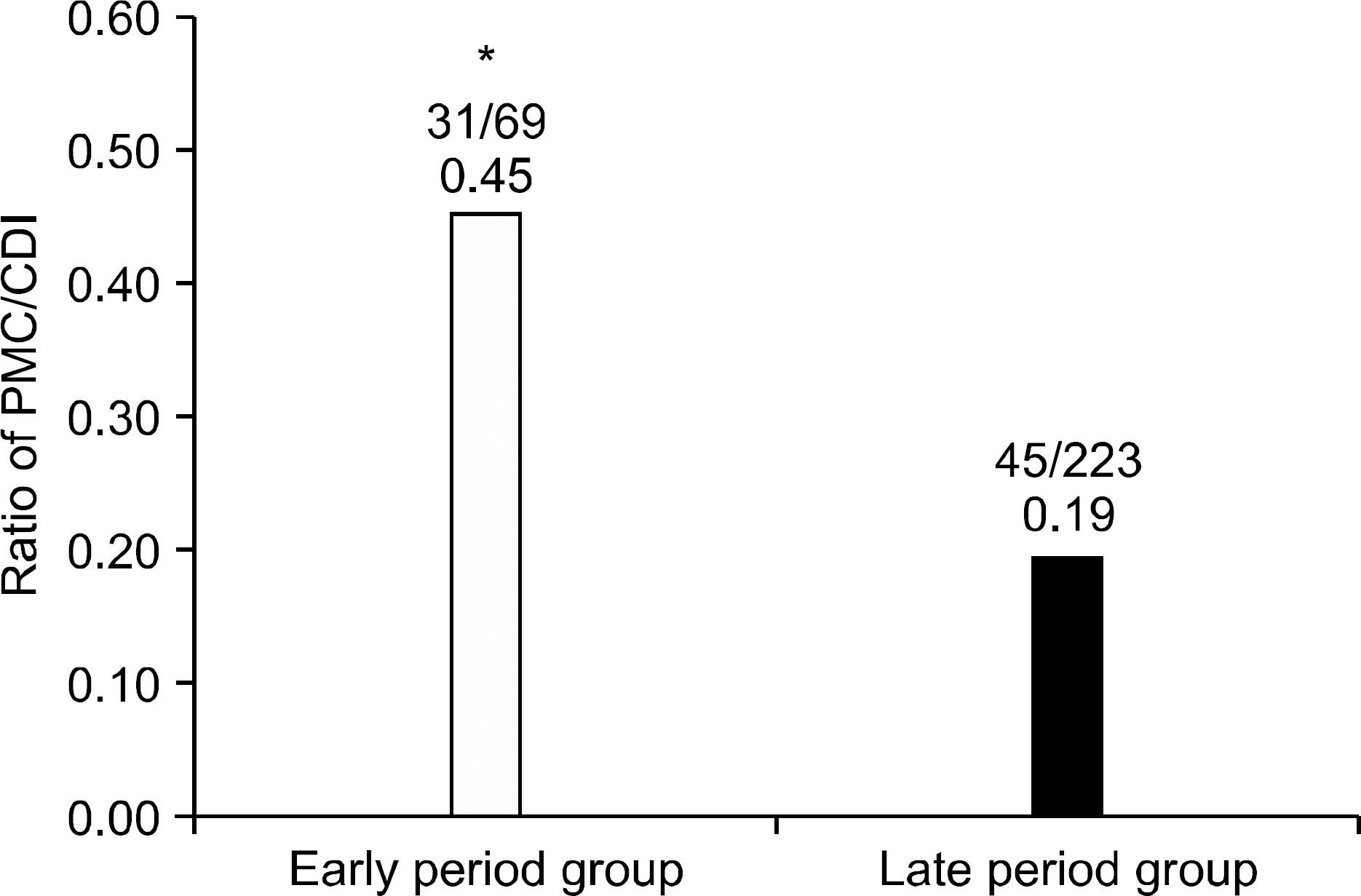
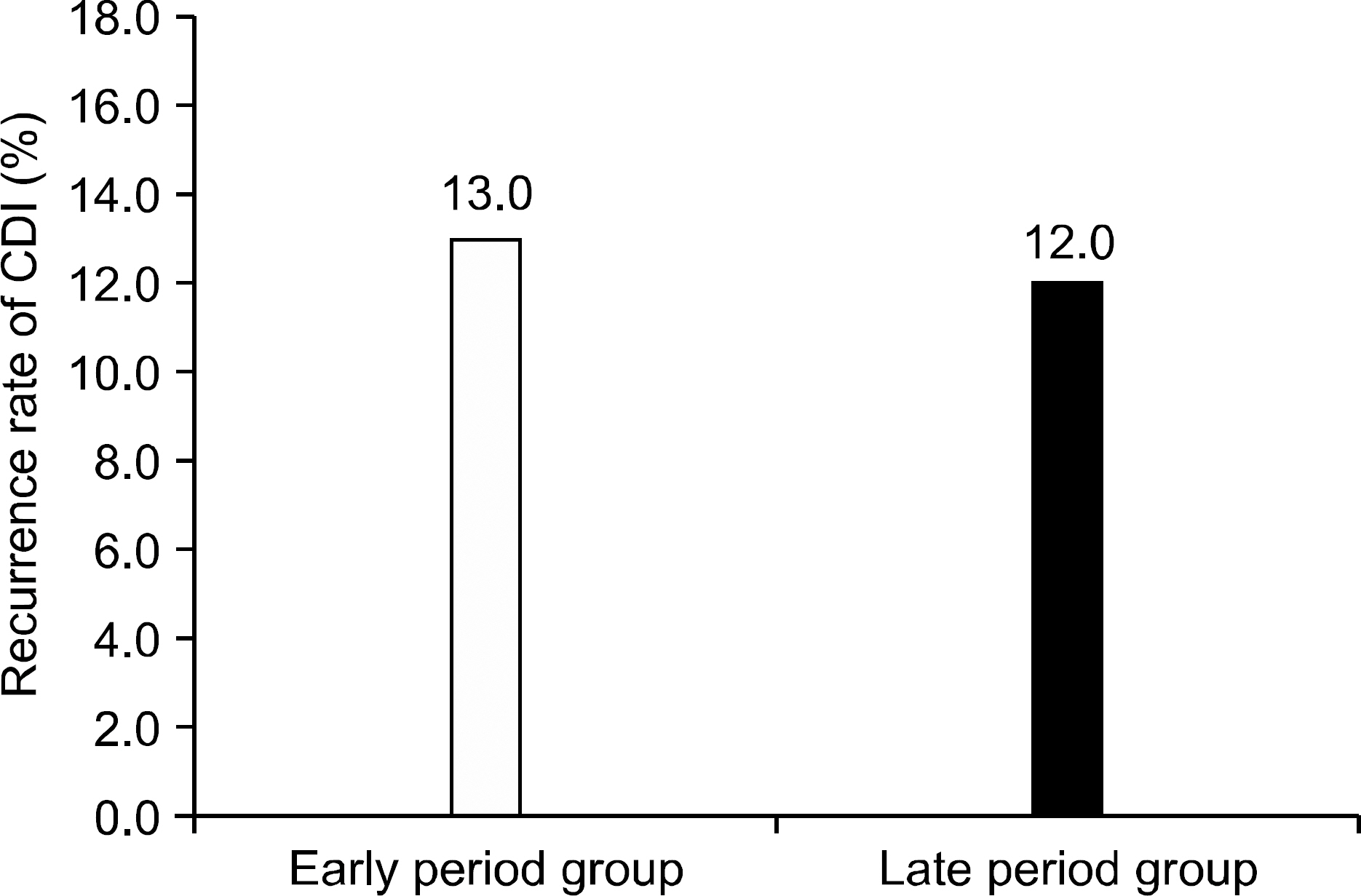
The incidence of CDI was 21.73 cases per 10,000 admitted patients in early period group, and significantly increased to 71.71 cases per 10,000 admitted patients in late period group (p<0.01). The hospital stay duration at the time of CDI diagnosis was shorter in late period group. Cephalosporin had the highest ratio as the causative antibiotics of CDI. However, there was no difference in recurrence rate between early and late period groups. Recurrence associated clinical factor was serum albumin level.
CONCLUSIONS
The incidence of CDI showed increasing tendency during recent 6 years. The awareness of increasing disease burden is the first step in control of CDI.
MeSH Terms
Figure
Cited by 3 articles
-
Epidemiology and Clinical Characteristics of Clostridium difficile-associated Disease in Children: Comparison between Community- and Hospital-acquired Infections
Hye Jung Cho, Eell Ryoo, Yong Han Sun, Kang Ho Cho, Dong Woo Son, Hann Tchah
Korean J Pediatr Gastroenterol Nutr. 2010;13(2):146-153. doi: 10.5223/kjpgn.2010.13.2.146.Long-Term Clinical Outcome of
Clostridium difficile Infection in Hospitalized Patients: A Single Center Study
Young Seok Doh, You Sun Kim, Hye Jin Jung, Young Il Park, Jin Won Mo, Hyun Sung, Kyung Jin Lee, Young Ki Seo, Jeong Seop Moon, Seong Woo Hong
Intest Res. 2014;12(4):299-305. doi: 10.5217/ir.2014.12.4.299.Refractory pseudomembranous colitis that was treated successfully with colonoscopic fecal microbial transplantation
Jun Young Shin, Eun Jung Ko, Seung Ho Lee, Jong Bum Shin, Shin Il Kim, Kye Sook Kwon, Hyung Gil Kim, Yong Woon Shin, Byoung Wook Bang
Intest Res. 2016;14(1):83-88. doi: 10.5217/ir.2016.14.1.83.
Reference
-
1. Fekety R, Shah AB. Diagnosis and treatment of Clostridium difficile colitis. JAMA. 1993; 269:71–75.2. Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994; 330:257–262.3. Monaghan T, Boswell T, Mahida YR. Recent advances in Clostridium difficile-associated disease. Gut. 2008; 57:850–860.4. DuPont HL, Garey K, Caeiro JP, Jiang ZD. New advances in Clostridium difficile infection: changing epidemiology, diagnosis, treatment and control. Curr Opin Infect Dis. 2008; 21:500–507.5. Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007; 142:624–631.6. Blossom DB, McDonald LC. The challenges posed by re-emerging Clostridium difficile infection. Clin Infect Dis. 2007; 45:222–227.7. Lee CR, Lee JK, Cho YS, Yoo HM, Kim WH, Lee KW. A clinical investigation of Clostridium difficile-associated disease. Korean J Gastroenterol. 1999; 33:338–347.8. Chung JW, Byeon JS, Choi KS, et al. Usefulness of sigmoidoscopy in pseudomembranous colitis: focused on the comparison with immunological assay for C. difficile toxin and the role as predictive factor for clinical outcome. Intest Res. 2007; 5:45–51.9. Jumaa P, Wren B, Tabaqchali S. Epidemiology and typing of Clostridium difficile. Eur J Gastroenterol Hepatol. 1996; 8:1035–1040.10. Lee JK, Cho JY, Kim YS, et al. Comparative value of sigmoidoscopy and stool cytotoxin-A assay for diagnosis of pseudomembranous colitis. Intest Res. 2005; 3:61–67.11. Biller P, Shank B, Lind L, et al. Moxifloxacin therapy as a risk factor for Clostridium difficile-associated disease during an outbreak: attempts to control a new epidemic strain. Infect Contol Hosp Epidemiol. 2007; 28:198–201.12. Kato H, Ito Y, van den Berg RJ, Kuijper EJ, Arakawa Y. First isolation of Clostridium difficile 027 in Japan. Euro Surveil. 2007; 12:E070111. .3.13. Barbut F, Decré D, Lalande V, et al. Clinical features of Clostridium difficile-associated diarrhoea due to binary toxin (actin-specific ADP-ribosyltransferase)-producing strains. J Med Microbial. 2005; 54:181–185.14. Roberts MC, McFarland LV, Mullany P, Mulligan ME. Characterization of the genetic basis of antibiotic resistance in Clostridium difficile. J Antimicrob Chemother. 1994; 33:419–429.15. Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Euroupe. Lancet. 2005; 366:1079–1084.16. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silvar J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hospital Epidemiol. 1995; 16:459–477.17. Pierce PF Jr, Wilson R, Silva J Jr, et al. Antibiotic-associated pseudomembranous colitis: an epidemiologic investigation of a cluster of cases. J Infect Dis. 1982; 145:269–274.
Article18. Tae CH, Jung SA, Song HJ, et al. The first case of anti-biotic-associated colitis by Clostridium-difficile PCR ribotype 027 in Korea. J Korean Med Sci. 2009; 24:520–524.19. O'Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009; 136:1913–1924.20. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005; 40:1586–1590.21. Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005; 353:2442–2449.22. Ramaswamy R, Grover H, Corpus M, Daniels P, Pitchumoni CS. Prognostic criteria in Clostridium difficile colitis. Am J Gastroenterol. 1996; 91:460–464.23. Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007; 102:2047–2057.
Article24. Jump RL, Puitz MJ, Donskey CJ. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to ex-plain the associations between proton pump inhibitors and C difficile-associated diarrhea? Antimicrob Agents Chemother. 2007; 51:2883–2887.25. Shin BM, Kuak EY, Yoo HM, et al. Multicentre study of the prevalence of toxigenic Clostridium difficile in Korea: results of a retrospective study 2000-2005. J Mel Microbiol. 2008; 57:697–701.26. Johal SS, Hammond J, Solomon K, James PD, Mahida YR. Clostridium difficile associated diarrhoea in hospitalised patients: onset in the community and hospital and role of flexi-ble sigmoidoscopy. Gut. 2004; 53:673–677.27. Gebhard RL, Gerding DN, Olson MM, et al. Clinical and endoscopic findings in patients early in the course of Clostridium difficile-associated pseudomembranous colitis. Am J Med. 1985; 78:45–48.28. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomized trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983; 2:1043–1046.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clostridium difficile Infection: What's New?
- Clostridium difficile Infection: A Worldwide Disease
- A Case of Reactive Arthritis in a Patient with Clostridium Difficile Diarrhea
- Review of clinical and laboratory features of patients to determinethe significance of increased isolation of clostridium difficile
- New Treatment Option for Recurrent Clostridium difficile Infection